Not only women, but also many men in adulthood want to look beautiful. From early childhood and adolescence, girls spend a lot of time in front of the mirror - bringing beauty to their faces. Wrinkles are the real enemy of women's youth and beauty. However, in the category of modern cosmetic preparations, you can easily find exactly the product that will help you quickly get rid of this problem. One such remedy is Dysport. It is the most productive means of aesthetic cosmetology, a good alternative to plastic surgery. The toxin, which has become a means of rejuvenation, now provides an excellent opportunity to look attractive at any age.
Pharmacological properties of the drug Dysport
The botulinum toxin type A complex blocks the release of acetylcholine at the neuromuscular junction, which leads to the elimination of muscle spasm at the injection site. The effect is observed for 3–4 months after administration. The resumption of nerve impulse transmission occurs gradually as new nerve endings are formed and contacts with the postsynaptic motor terminal plate are restored. Studying the pharmacokinetics of botulinum toxin is difficult due to its high toxicity, large molecular weight, low doses used, and difficulty in applying a radioactive label. After intramuscular administration, the reaction delay time is 2–3 days, and the maximum effect is observed between the 10th and 21st days. The average duration of action is 8–12 weeks.
Restrictions and contraindications
Before Dysport injections, you must consult with a specialist who will give an opinion on the use of the drug and conduct tests to identify existing diseases and pathologies of the body.
This is necessary in order to make a decision about using the drug. Relative contraindications include:
- Pregnancy and breastfeeding period.
- Exacerbation of chronic diseases, acute diseases.
- Skin lesions in the area of drug administration.
Absolute contraindications:
- Allergic reactions to the components of the drug.
- Some cardiovascular pathologies.
- Autoimmune neuromuscular diseases.
- Respiratory failure.
- Swallowing dysfunction.
- Some endocrine diseases.
Botulinum toxin injections are most effective for facial wrinkles. After 35 years, age-related changes in the structure of the skin begin, the intensity of collagen production decreases, tissue microcirculation worsens, and regenerative processes slow down. To combat natural changes, it is recommended to combine Dysport with anti-aging drugs.
Indications for use of the drug Dysport
- blepharospasm;
- hemifacial spasm;
- spastic torticollis;
- spasticity of the arm after a stroke;
- spastic equinus foot deformity in adults after stroke;
- hyperkinetic (expressive) facial wrinkles in adults;
- dynamic deformation of the foot of spastic origin in children with cerebral palsy;
- anal fissure;
- local hyperhidrosis (palms, feet, armpits);
- tension headache;
- esophageal achalasia;
- hyperreflexia of the bladder.
Use of the drug Dysport
Dysport units are specific to this drug and cannot be compared with other botulinum toxin type A preparations. Treatment with Dysport can only be carried out by a specialist who has been trained in therapy with this drug. The central part of the rubber stopper of the bottle should be treated with alcohol immediately before puncture. For the puncture, a sterile needle of size 23 or 25 is used. A needle of size 29–30 is used to correct facial wrinkles, and a needle of size 27 is used for injection for anal fissure. Treatment of bilateral and unilateral blepharospasm, hemifacial spasm The lyophilisate is dissolved in 2.5 ml of 0.9% sodium chloride solution for injection, resulting in a transparent white solution containing 200 units in 1 ml. After preparation, the solution is suitable for use within 8 hours. The total initial dose for the treatment of bilateral blepharospasm is 120 IU for each eye. The drug is administered subcutaneously in a dose of 0.1 ml (20 units) medially, in a dose of 0.2 ml (40 units) laterally into the connection between the preseptal and orbital parts of both the inferior and superior orbital muscles of each eye. For injections into the upper eyelid, the needle should be directed away from the center so as not to touch the muscle that lifts the eyelid. The manifestation of the clinical effect can be expected within 2–4 days, the maximum therapeutic effect develops by the end of the 2nd week. To prevent relapse, administration of the drug should be repeated every 8 weeks or depending on the clinical situation. With each subsequent administration, the dose should be reduced to 80 units per eye (for example, 0.1 ml (20 units) medially and 0.1 ml (20 units) laterally above and below the eye according to the scheme presented above). In the future, the dose of the drug can be reduced to 60 units per eye, by eliminating the introduction medially into the lower eyelid. Subsequent doses are determined by the doctor depending on the effect obtained. With unilateral blepharospasm, treatment is limited to the area of the affected eye. For hemifacial spasm, treatment is the same as for unilateral blepharospasm. The drug in the recommended dose is administered to adults, regardless of age, including elderly people. Spasmodic torticollis The drug is dissolved in 1 ml of 0.9% sodium chloride solution for injection, obtaining a solution containing 500 units in 1 ml. The drug is administered intramuscularly. The dose for the treatment of torticollis in adult patients with normal body weight and satisfactorily developed neck muscles is established regardless of age. The dose is reduced in case of significant decrease in body weight, including in elderly people. The recommended starting dose for spastic torticollis is 500 units. It is injected in parts into the 2-3 most active muscles of the neck. For rotational torticollis, 500 units are administered as follows: 350 units into the splenius capitis muscle, ipsilateral in the direction of chin/head rotation, and 150 units into the sternocleidomastoid muscle, contralateral to the direction of rotation. For lateral torticollis, the drug in a dose of 500 units is distributed as follows: 350 units are injected ipsilaterally into the splenius capitis muscle and 150 units ipsilaterally into the sternocleidomastoid muscle. Cases that involve elevation of the shoulder by the trapezius or levator scapulae muscles may require treatment according to visible muscle hypertrophy or electromyographic examination. If it is necessary to inject into 3 muscles, a dose of 500 units is distributed as follows: 300 units of the drug are injected into the splenius muscle, 100 units into the sternocleidomastoid muscle and 100 units into the third muscle. For posterior torticollis, a dose of 500 units is distributed as follows: 250 units in each splenius muscle of the head. Then the drug can be injected bilaterally into the trapezius muscles (250 units per muscle). If after the 6th week the effect is insufficient, repeated injections can be performed. Bilateral injections into the splenius muscles may increase the risk of neck muscle weakness. Treatment of all other forms of torticollis is directly related to the knowledge of the specialist and the electromyography data that he uses. Electromyography should be used for all complex forms of torticollis, in case of repeated injections or after unsuccessful injections. Electromyography is used when the drug is administered into deep-lying muscles in patients with excess body weight, with muscles that are poorly defined by palpation. With subsequent administrations, the dose of the drug can be optimized depending on the clinical effect and taking into account adverse reactions. It is recommended to use the drug in doses of 250–1000 units; Higher doses are also used, but they can cause an increase in side effects, in particular the appearance of dysphagia. The drug is not recommended for use in doses exceeding 1000 units. Improvement in spastic torticollis occurs within 1 day after injection. Injections should be repeated every 8-12 weeks to prevent relapses. Spasticity of the arm in adults after a stroke 1 ml of 0.9% sodium chloride solution for injection is injected into the bottle with the drug, obtaining a solution containing 500 IU of Dysport in 1 ml. The drug is administered intramuscularly at five points. The maximum dose used is 1000 units, which is distributed between five muscles: m. flexor digitorium profundus (FDP), m. flexor digitorium superficialis (FDS), m. flexor carpi ulnaris (FCU), m. flexor carpi radialis (FCR) and biceps brachii (BB). When choosing a site for injection, you should be guided by the anatomical location of the muscles; the immediate injection site is determined by palpation. In all muscles, except the biceps brachii, the injection is made at one point. The injection into the biceps brachii muscle is performed at two points. Recommended doses are shown in the table.
Muscle | BB | FDP | FDS | FCU | FCR | Total dose |
| Dose of Dysport, ED | 300–400 | 150 | 150–250 | 150 | 150 | 1000 |
The initial total dose of the drug may be reduced to 500 units to avoid excessive weakness of the muscles being injected if the muscles are small, if the BB muscle is not being injected, or if the patient is being injected at multiple sites in the same muscle. Injections can be repeated approximately every 16 weeks or if necessary to maintain the effect, but not more than once every 12 weeks. Spastic equinus deformity of the foot in adults after a stroke: 1 ml of 0.9% NaCl is injected into the bottle with the drug and a solution containing 500 units in 1 ml is obtained. The drug is administered intramuscularly. The recommended dose of the drug, 1500 units, is distributed between the muscles of the gastrocnemius and m.soleus, but an injection can also be made into the m.tibialis posterior. EMG data can be used to determine the most active muscles. The initial dose may be reduced if it is likely that the dose may cause increased weakness of the muscles being injected, if the patient's target muscles are small, or if the patient needs to have other muscle groups injected at the same time. Clinical improvement can be observed within 2 weeks after injection. Injections can be repeated approximately every 16 weeks or if necessary to maintain the effect, but not earlier than after 12 weeks. Hyperkinetic (expressive) facial wrinkles To correct vertical wrinkles in the area between the eyebrows, injections of the drug are made into the m. corrugator supercilii 10–20 units in 2–4 points, and in m. procerus 5–10 units in 2 points. The total dose is 30–100 units. Frontal region Elimination of hyperkinetic wrinkles in the frontal region is carried out by injecting the drug into the area of greatest tension m. frontalis. The number of injection points can be arbitrary. All of them should be located 2 cm above the level of the eyebrows on the same line or in a V-shape. The total dose is 20–90 units of Dysport, based on the calculation of 5–15 units per point, the total number of points is 4–6. Area of the outer corner of the eye Correction of wrinkles in the area of the outer corner of the eye (“crow’s feet”) is carried out by subcutaneous injection into points located 1 cm lateral to the outer corner of the eye, at the rate of 5–15 units of Dysport per injection point. The number of points is from 2 to 4 for each eye. The maximum recommended total dose is 120 units. The frequency of repeated injections depends on the timing of restoration of facial muscle activity. The duration of the effect is 3–4 months. If an adequate dose of the drug was administered during the first injection, the total dose during the second and subsequent injections can be reduced to 15–20 units for the appropriate areas. However, there may be an increase in the period of action of the drug to 6–9 months. If the initial dose of the drug was insufficient, then with subsequent injections it should be increased. Area of the back of the nose To correct wrinkles on the back of the nose, injections are made into the middle of the nasal muscles. The dose is distributed at 5-10 units at 1-2 points in each muscle. The muscle relaxant effect of Dysport on the facial muscles of the face is clinically manifested on the 2-3rd day after administration and reaches a maximum on the 14-15th day. Doses of Dysport used in aesthetic medicine do not cause the development of systemic side effects. Dynamic deformation of the foot caused by spasticity in children with cerebral palsy The contents of the bottle are dissolved in 1 ml of 0.9% sodium chloride solution for injection, obtaining a solution containing 500 units in 1 ml. The drug is injected intramuscularly into the calf muscles. The initial dose is 20 units/kg of the child's body weight - distributed equally for injection into the calf muscles. If only one calf muscle is affected, a dose of 10 units per 1 kg of body weight is administered into it. The drug is administered mainly into the m. gastrocnemius, however, it is possible to introduce it into m. soleus and m. tibialis posterior. It is possible to use the electromyography method to determine the most active muscles. The initial dose of the drug is reduced to prevent excessive weakness of the muscles into which the drug is injected if the muscle is small or if the injection is performed at several points in the same muscle. The optimal dose is determined individually. Depending on the results of the initial dose, subsequent doses of the drug may range from 10-30 units/kg, distributed to the muscles of both legs. To prevent side effects, the total dose should not exceed 1000 units. Clinical improvement occurs within 2 weeks after drug administration. Injections can be repeated approximately every 16 weeks or more often if necessary to maintain the effect, but not more often than after 12 weeks. Anal fissure The contents of the bottle are dissolved in 2.5 ml of 0.9% sodium chloride solution for injection, obtaining a solution that contains 200 units in 1 ml. First, the internal anal sphincter is palpated, and then an injection is performed using a 27-size needle on both sides near the fissure (30 units of the drug). To treat one patient, 60 units of the drug Dysport are used. The administration of Dysport into the internal anal sphincter does not require the use of sedatives or local anesthesia and does not cause any side effects or irreversible damage to the sphincter. Local hyperhidrosis (palms, feet, armpits) The contents of the bottle are dissolved in 5 ml of 0.9% sodium chloride solution for injection, obtaining a solution that contains 100 units in 1 ml. Before injections, it is necessary to prepare the patient: shave the hair in the armpits, in the foot area the patient must have a pedicure (to remove areas of hyperkeratosis in the foot area). When performing injections in the palm area, no special preparation is required. The specific localization of hyperhidrosis, its area, intensity and features of the distribution of zones of hyperhidrosis are preliminarily determined based on the patient’s subjective complaints, clinical examination and using the Minor test (iodine-starch test, which is based on the color reaction of iodine with starch, carried out only in the presence of water - in the zone of hyperhidrosis ). If the patient has increased pain sensitivity, local anesthesia is performed before the injection. The minimum dose of Dysport required to eliminate hyperhidrosis on a skin area of 1 cm2 is 1.5–2 units. Taking this into account and having determined the area of hyperhidrosis, the dose of the drug is determined. Injections are made at a distance of 2 cm from one another, so the density of drug injection points is 1 point per 4 cm2. The drug is administered to each point in a dose of 6–8 units. Improvement in symptoms is observed already on the 2nd day after injection and reaches its greatest severity on the 5th day after injection. The duration of the drug varies between 4 and 8 months. Tension headache The contents of the bottle are dissolved in 5 ml of 0.9% sodium chloride for injection, obtaining a solution containing 100 units in 1 ml. Equal doses of 25 units (0.25 ml) are administered on both sides into the frontal, temporal, occipital and sternocleidomastoid muscles, the total dose of the administered drug is 200 units. The improvement in the condition reaches its greatest severity on the 5th day after administration, the duration of the effect is at least 4 weeks. Esophageal achalasia The contents of the bottle are dissolved in 0.9% sodium chloride solution for injection, obtaining a solution containing 60 units in 1 ml. In each of the four quadrants of the lower sphincter of the esophagus, using endoscopy, using a needle that is used for sclerosing therapy in angiosurgery (volume 0.5 mm), 2 injections of 0.5 ml of the drug Dysport are performed (concentration of the solution is 30 U/ml ) at a distance of 1 cm from one another. The total dose per patient is 240 units. Improvement is noted on the 7th day. The effect of the drug lasts from 6 to 12 months. Hyperreflexia of the bladder The contents of the bottle are dissolved in 5 ml of 0.9% sodium chloride for injection to obtain a solution containing 100 units in 1 ml. Injections are performed with a 23-gauge endoscopic needle, short-term anesthesia is performed, and sonographic monitoring of the thickness of the bladder wall is carried out.
- For neurogenic overactive bladder, injections are made into 20–40 detrusor points, except the trigonum, from 25 to 40 units per point. The total dose is 800–1000 units of Dysport per patient.
- For non-neurogenic overactive bladder, injections are made into 20 detrusor points, from 15 to 25 units per point, except the trigonum. The total dose is 300–500 units of Dysport per patient.
The positive effect of the drug lasts from 6 to 10 months.
Side effects of the drug Dysport
Blepharospasm and hemifacial spasm Side effects can be observed if the doctor does not comply with the injection rules (errors in dilution, accurate dose calculation) and the associated excessive diffusion of the drug into a group of muscles located nearby. It is also necessary to take into account the anatomical and physiological characteristics of the muscles into which the injection is performed. Blepharoptosis is the most common side effect of the drug. Several patients experienced diplopia or symptoms that indicated the spread of the paralytic effect to the middle muscles of the face. These phenomena disappear within 2–4 weeks after stopping treatment with the drug. Dry eyes and the development of keratitis may occur due to a decrease in blinking frequency. In such cases, the use of artificial tears is recommended. Minor and transient hematomas and swelling of the eyelids may occur. Reversible external ophthalmoplegia may also occur when the drug is used in high doses. Injections may be accompanied by a burning sensation at the injection site for 1–2 minutes. Very rarely, allergic reactions may develop in the form of skin rashes and flu-like symptoms. Spasmodic torticollis Side effects may occur due to deep injections or injections made at incorrectly selected points, which leads to temporary excessive weakness of the muscles located nearby. The most common side effect during treatment of spasmodic torticollis is dysphagia. In a double-blind, placebo-controlled study, dysphagia was observed in 29% of patients who received Dysport at a dose of 500 units and in 10% of patients who received placebo. This side effect is dose-dependent and most often occurs with injections into the sternocleidomastoid muscle. If dysphagia occurs, the patient should refrain from eating rough food until symptoms disappear completely. In patients with severe manifestations of this side effect, a large accumulation of saliva was observed during laryngoscopy. Sometimes in such cases, aspiration is required, especially in patients with impaired respiratory function. Manifestations such as weakness of the neck muscles, dry mouth, and change in voice are also possible. In isolated cases, general weakness and visual impairment (including diplopia and blurred vision) are observed. Breathing problems may occur if the drug is used in high doses. These side effects disappear within 2–4 weeks. Allergic reactions (skin rash and flu-like symptoms) may also occur. The appearance of anti-botulinum antibodies has been noted in some patients. The clinical manifestation of this fact was a decrease in the therapeutic effect, which required an increase in the dose of the drug. Arm spasticity in adults after stroke The most common side effects are weakness of the muscles located near the injection site (6.7%), flu-like symptoms (5.6%), fatigue (3.3%), pain at the injection site (3. 3%), general weakness (2.2%), drowsiness (1.1%). Most of these phenomena disappear within 2 weeks. Spastic equinus foot deformity in adults after stroke: In 10 clinical studies involving approximately 1100 patients who received Dysport, the following side effects were reported: dysphagia, leg muscle weakness, urinary incontinence, gait disturbance, accidental injuries due to falls. Hyperkinetic (expressive) facial wrinkles Possible temporary ptosis of the upper eyelid (3%), numbness, pain at the injection site, headache (1.3%), hematoma at the injection site (3–10%), drooping or raising of the eyebrows ( ≤1%). Diplopia occurs very rarely. The most significant side effect is ptosis of the upper eyelid, which is caused by the diffusion of the drug into the muscle that lifts the upper eyelid when correcting vertical wrinkles on the bridge of the nose or horizontal wrinkles on the forehead. Ptosis is reversible and disappears within 3–4 weeks. Minimizing the risk of side effects, as well as their severity, is possible only through strict adherence to the method of administration of the drug Dysport when correcting hyperkinetic (expressive) facial wrinkles, taking into account the anatomical and physiological characteristics of each patient. Dynamic Foot Deformity Caused by Spasticity in Children with Cerebral Palsy Side effects were studied in three studies involving 142 patients treated with Dysport and 75 patients treated with placebo. With a frequency of more than 5%, pain in the legs (8%), pharyngitis (8%), accidental injuries due to a fall (7%), bronchitis (6%), and increased body temperature (6%) were noted. With a frequency of 1–5%, viral infections (5%), other infections (4%), rhinitis (4%), convulsions (4%), upper respiratory tract infections (4%), asthenia (3%), asthma ( 3%), cough (3%), vomiting (3%), colds (2%), diarrhea (2%), urinary incontinence (2%), gastroenteritis (1%), laryngitis (1%), drowsiness ( 1%). Most of these side effects were also observed in the placebo group, indicating that these symptoms are common to the pediatric patient population as a whole. The incidence of seizures in the Dysport group was identical to that in the placebo group, further supporting the idea that seizures are the most common complication in patients with cerebral palsy. Asthenia and urinary incontinence in patients in the group receiving Dysport were associated with the use of the drug in higher doses (20–30 U/kg) and could occur due to systemic distribution of the drug. A small number of patients experienced localized skeletal muscle weakness, which sometimes resulted in falls. Most of these symptoms disappeared within 2 weeks. Anal fissure No side effects or irreversible changes in the sphincter were observed when following the method of using the drug Dysport. Local hyperhidrosis There are no side effects or complications if the recommendations for dosing of the drug and injection technique are followed. Temporary side effects, which are very rare, include pain at the injection site and microhematoma for 2-5 days after injection. Tension headache Very rarely, weakness of the neck muscles can be observed, which goes away within 1-2 weeks. Esophageal achalasia Up to 75% of patients may experience mild intermittent chest pain during the injection. Cases of gastroesophageal reflux have also been noted (in 0–13% of cases). Hyperreflexia of the bladder No side effects are observed if the method of using the drug Dysport is followed.
Preparation and rehabilitation
Injections require little preparation:
- You should not drink alcohol the day before.
- On the day of the procedure, you must limit your mobility, do not make sudden movements, or bend over.
After injections:
- Do not take a horizontal position for several hours.
- Avoid sudden temperature changes.
- Don't exercise for a few days.
- Avoid stress on the heart.
- Avoid drinking alcohol for two weeks.
Special instructions for the use of the drug Dysport
Warning and safety precautions. Cases of side effects have been reported that resulted from distant spread of the action of botulinum toxin from the injection site. Patients receiving therapeutic doses of the drug may experience severe muscle weakness. The risk of such side effects may be reduced by using the lowest effective dose and not exceeding recommended doses. Treatment with Dysport should only be carried out by a specialist who has experience in diagnosing and treating such conditions and has been trained in the use of Dysport. The drug is used with caution and under strict medical supervision in patients with subclinical and clinical manifestations of neuromuscular conduction disorders. Such patients may have increased sensitivity to drugs such as Dysport, which may manifest as severe muscle weakness. Patients with impaired neuromuscular transmission, difficulty swallowing and breathing are at higher risk for these effects. Treatment of such patients should be carried out under the supervision of a specialist in cases where the expected benefit of treatment outweighs the risk. It is necessary to warn patients and those accompanying them about the need for emergency assistance in case of problems with swallowing, speech and breathing problems. In children with dynamic foot deformity caused by spasticity due to cerebral palsy, the drug can be used starting from 2 years of age. Instructions for processing drug residues. Immediately after the injection, the remaining solution in the vial and syringe should be inactivated with diluted sodium hypochlorite solution (containing 1% active chlorine). All ancillary materials that have been in contact with the drug must be disposed of in accordance with hospital practice standards. Spilled medication should be removed using an absorbent cloth moistened with diluted sodium hypochlorite solution.
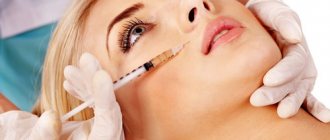
How does the procedure work?
Injections do not cause intense pain in most patients; anesthesia is not used for injections.
- The cosmetologist marks out places for the injection of Dysport and disinfects the skin.
- The drug is administered with a thin needle in a calculated dosage.
- A cold compress is applied to the injection area.
- After removing the compress, the patient is allowed to go home.
The first effect becomes noticeable within three days. The pronounced result is visible within one and a half to two weeks from the moment of the procedure.