Terbinafine
The drug is generally well tolerated. Side effects are usually mild or moderate and transient.
When assessing the frequency of side effects, the following gradations were used: “very often” (≥ 1/10), “often” (≥1/100 <1/10), “infrequently” (≥1/1000 <1/100), “ rarely" (≥1/10000 <1/1000), "very rarely" (<1/10000), including isolated reports.
Disorders of the hematopoietic system: infrequently - anemia, very rarely - neutropenia, agranulocytosis, thrombocytopenia, pancytopenia.
Immune system disorders: very rarely - anaphylactoid reactions (including angioedema), cutaneous and systemic lupus erythematosus (or their exacerbation).
Nervous system disorders: very often - headache; often - dizziness, disturbances in taste, up to their loss (usually recovery occurs within a few weeks after stopping treatment); infrequently - paresthesia, hypoesthesia. There are isolated reports of cases of long-term disturbances in taste. In some cases, while taking the drug, exhaustion was noted.
Mental disorders: often - depression; infrequently - anxiety.
Visual disturbances: infrequently - visual disturbances.
Hearing and labyrinthine disorders: uncommon - tinnitus.
Disorders of the liver and biliary tract: rarely - hepatobiliary dysfunction (mainly of cholestatic nature), incl. liver failure, including very rare cases of severe liver failure (some fatal or requiring liver transplantation; in most cases where liver failure developed, the patients had serious underlying systemic diseases and the causal relationship of liver failure to terbinafine was questionable), hepatitis, jaundice, cholestasis, increased activity of liver enzymes.
Disorders of the digestive system: very often - bloating, loss of appetite, dyspepsia, nausea, mild abdominal pain, diarrhea.
Disorders of the skin and subcutaneous tissues: very often - rash, urticaria, infrequently - photosensitivity reactions; very rarely - Stevens-Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, erythema multiforme, toxic skin rash, exfoliative dermatitis, bullous dermatitis, psoriasis-like skin rashes or exacerbations of psoriasis, alopecia.
Musculoskeletal and connective tissue disorders: very often - arthralgia, myalgia.
General disorders: often - feeling of fatigue; infrequently - increased body temperature.
Laboratory and instrumental data: infrequently - loss of body weight (secondary to a disturbance in the sense of taste).
Based on spontaneous reports received during the post-registration period and literature data, the following adverse events were identified, the frequency of which, due to the inaccurate number of patients, cannot be established:
Immune system disorders: anaphylactic reactions, serum sickness-like syndrome.
Visual disturbances: blurred vision, decreased visual acuity.
Skin and subcutaneous tissue disorders: drug rash with eosinophilia and systemic symptoms (rash, swelling, fever, and swollen lymph nodes).
Hearing and labyrinthine disorders: hearing loss, hearing impairment.
Vascular disorders: vasculitis.
Nervous system disorders: loss of smell, including for a long period of time, decreased sense of smell.
Digestive system disorders: pancreatitis.
Disorder of the musculoskeletal and connective tissue side: rhabdomyolysis.
General disorders: influenza-like syndrome.
Blood and lymphatic system disorders: anemia.
Laboratory and instrumental data: increased activity of creatine phosphokinase in blood plasma.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
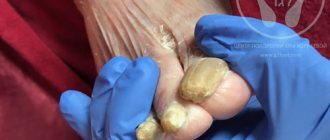
Modern possibilities of using terbinafine for the treatment of fungal diseases
The incidence rate increases significantly in patients in older age groups, regardless of gender. According to foreign researchers, onychomycosis affects from 2 to 18.5% of the total population of the planet, and in the age group of 70 years and older, 50% of the world’s population is affected by this disease [1,8]. According to various authors, fungal infections of the feet are observed in a third of all European residents [18,20]. As a rule, the development of onychomycosis is preceded by mycosis of the feet - according to our data, only 30% of patients had isolated onychomycosis, in the remaining 70% of patients it was combined with mycosis, which began before the fungal infection of the nails [2]. Thus, treatment of mycosis of the feet is the prevention of the development of onychomycosis. Very often, dermatologists are faced with insufficient awareness of patients - the latter do not know about the presence of a fungal infection, even seeing manifestations on the skin in the form of peeling, intertrigo, hyperkeratosis, and attribute this to physiological characteristics or age-related changes. On the other hand, some patients, seeing changes in the nail plates, regard this as onychomycosis and begin to treat it themselves with all available means. In the presence of mycosis of smooth skin without affecting the nails and involving the hair in the process, a cure can be achieved using only topical antimycotics. One of the most active antimycotics is terbinafine. It belongs to allylamine derivatives. In terms of the breadth of the spectrum of antifungal action, this drug surpasses all other antifungal drugs: polyene antibiotics, azoles (imidazole derivatives - clotrimazole, ketoconazole, etc., as well as first generation triazoles - itraconazole, fluconazole, etc.) and echinocandins. In recent years, many studies have been conducted confirming the high effectiveness and safety of terbinafine (Lamisil) in the treatment of fungal infections. The undoubted advantages of this drug include a large number of its forms for external use - cream, dermgel, spray and film-forming solution for the treatment of types of mycosis. Terbinafine in the form of a cream is ideal for squamous and squamous-hyperkeratotic forms. In the first case, it is enough to apply the cream to the affected area once a day. within 7 days [15]. For the treatment of hyperkeratotic forms of mycosis, use the drug 2 times a day. within 2 weeks. makes it possible to achieve clinical and mycological cure. In a study conducted by Yu.N. Perlamutrov and K.B. Olkhovskaya, it has been shown that complete mycological cure is achieved with the use of terbinafine 1 time per day. within 2 weeks. occurred in 80% of patients, and 2 times a day. – in 93%. At the same time, by the end of the course of treatment, the disappearance of peeling, itching and healing of deep and superficial cracks was observed in patients in both groups, but when using the cream 2 times a day. these symptoms resolved more quickly [4]. For intertriginous and dyshidrotic forms, it is appropriate to prescribe dermgel or spray - they have a pronounced antipruritic and drying effect. It is also convenient to prescribe the spray in the presence of lesions on smooth skin (lichen versicolor, microsporia) in the folds and on the scalp. So, according to A.A. Haldina et al., when applying both spray and dermgel to lesions in large folds, etiological cure was observed in 100% of cases, regardless of the nature of mycosis (Tr. rubrum, Ep. floccosum, C. albicans). When analyzing the dynamics of regression of skin symptoms, it was revealed that when using the spray, the regression of clinical symptoms is somewhat ahead of that when using dermgel, this is especially noticeable with the regression of itching and maceration [7]. There is a high effectiveness of using spray and dermgel for the treatment of patients with pityriasis versicolor. According to L.P. Kotrekhova et al., the effectiveness of dermgel and spray therapy was 94.8 and 93.8%, respectively. At the same time, patients note the ease of use and good organoleptic properties of the drug, such as the absence of greasiness and oily sheen of the skin. The hygroscopic properties of the drug made it possible to apply it to skin folds even in obese patients [3]. A new, and certainly promising form is a film-forming solution, which is applied to the skin of the feet once. N.N. Potekaev et al. treated 20 patients with various forms of mycosis of the feet with Lamisil Uno. Clinical and mycological cure occurred in 80% of patients by the 10th day of observation. The drug was ineffective in the squamous-hyperkeratotic form. For erased, squamous and intertriginous forms, the effectiveness is 100%. I would especially like to note that single use of the drug is very convenient for patients [5]. A randomized, double-blind, placebo-controlled study was conducted on the effectiveness of a single application of a 1% film-forming solution of terbinafine in 324 patients with mycosis of the feet. The result was assessed after 6 weeks. after using the drug. The study found negative culture, microscopy, and mycological cure rates of 91%, 81%, and 78%, respectively, while the placebo response rate was 17% [11]. Another very important point is the possibility of curing mycosis in a short time - with squamous and intertriginous forms of the lesion, the disease is cured in 1 week, while azole drugs must be used for 4 weeks. [17,18]. A longer course of therapy often leads to non-compliance with the treatment regimen; patients stop using the drug after visible improvements and are not treated further, which leads to relapse of the disease. In some cases (with damage to the nail plates, a common skin process, hair involvement), local antifungal therapy is not effective enough, and there is a need to prescribe a systemic drug, the most effective and safe of which is terbinafine [19]. Terbinafine has a fungicidal effect on dermatophytes, molds and some dimorphic fungi; on yeast fungi, depending on their type, it has a fungistatic or fungicidal effect [13,16]. The pronounced antimycotic effect of terbinafine also manifests itself in vivo, which is facilitated by its pharmacokinetics. After oral administration, the maximum concentration of terbinafine in the blood is reached within 2 hours, the drug is almost completely bound to plasma proteins. Due to its lipophilicity, terbinafine quickly diffuses through the dermis and accumulates in the stratum corneum and nail plates, and is also excreted with sebum, resulting in high concentrations of the drug in hair follicles and hair. Stable concentrations of terbinafine in tissues are achieved 10–14 days after the start of oral administration, the half-life of its elimination varies from 24 to 156 days, as a result of which the drug remains in the nail plates for a long time after stopping its use [8,10]. It is known that fungal infections of the scalp can be successfully treated with terbinafine. To confirm this, 3 groups of children aged 2 to 14 years with a confirmed diagnosis of trichophytosis were treated with griseofulvin for a month and terbinafine for 2 weeks. and 1 month It was shown that the effectiveness of therapy was approximately the same and amounted to 100% with griseofulvin, 95% with a 2-week course of terbinafine and 94.1% with a monthly dose [12]. A study was conducted on the effectiveness of oral terbinafine for the treatment of a hyperkeratotic form of mycosis of the feet with a torpid course. The drug at a dose of 125 mg was prescribed daily for 1 month. It was found that the maximum concentration of the drug (247.8 ng) in the stratum corneum of the epidermis was determined by the end of 1 week. treatment, which is 50 times the minimum inhibitory concentration for dermatophytes. After 6 weeks the concentration of the drug decreased to 50.73 ng, and after 8 weeks. terbinafine was no longer detectable in the skin. The effectiveness of therapy was 95% [14]. The low potential for drug interactions with other drugs is also important, because Onychomycosis often affects older people who are forced to take a large number of different medications for other diseases [13]. Thus, based on the analysis of literature data, we can conclude that terbinafine (Lamisil) is a highly effective drug for the treatment of fungal infections, has a large number of different forms, which significantly expand the possibilities of antifungal therapy. Literature 1. Malcolm B. Athlete's foot // Attending physician. 1998. No. 6. 2. Vasenova V.Yu. Immunopathogenesis, morphofunctional characteristics, clinical picture, complex therapy and prevention of onychomycosis: Abstract of thesis. ... doc. honey. Sci. M., 2008. 3. Kotrekhova L.P., Vasilyeva N.V., Raznatovsky K.I., Piotrovskaya I.V. Clinical efficacy and safety of terbinafine (Lamisil spray, Lamisil dermgel) in the treatment of pityriasis versicolor // Clinical dermatology and venereology. 2007. No. 3. P. 35–38. 4. Perlamutrov Yu.N., Olkhovskaya K.B. Optimization of therapy for mycoses of the feet in women using 1% Lamisil cream // Clinical dermatology and venereology. 2006. No. 2. P. 78–80. 5. Potekaev N.N., Serov D.G., Dvoryankova E.V., Zhukovsky R.O. Effective therapy of mycoses of the feet with a single use of a new external form of terbinafine - a film-forming solution of Lamisil Uno // Clinical Dermatology and Venereology. 2008. No. 4. pp. 85–88. 6. Sergeev A.Yu., Ivanov O.L., Sergeev A.Yu. and others. Study of modern epidemiology of onychomycosis // Bulletin of Dermatology and Venereology. 2002. No. 3. P. 31–35. 7. Khaldin A.A., Tsykin A.A., Izyumova I.M. Clinical and etiological effectiveness of 1% Lamisil® spray in the treatment of fungal infections of large skin folds // Russian Journal of Skin and Venereal Diseases. 2007. No. 1. P. 56. 8. Baran R. Onychomycosis: the current approach to diagnosis and therapy. London: Malden MA, 1999. 9. Chen SCA, Sorrell TC New Drugs, Old Drugs. Antifungal agents // Med. J. Austral. 2007. Vol. 187. No. 7. R. 404–409. 10. Cribier BJ, Bakshi R. Terbinafin in the treatment of onychomycosis: a review of its efficacy in high-risk populations and in patients with nondermatophyte infections // BJ Dermatol. 2004. Vol.150. R. 414–420. 11. De Chauvin MF, Viguie-Vallanet C., Kienzler JL, Larnier C. Novel, single-dose, topical treatment of tinea pedis using terbinafine: results of a dose-finding clinical trial. Mycoses. 2008 Jan. Vol. 51(1). R. 1–6. 12. Deng S., Hu H., Abliz P., Wan Z., Wang A., Cheng W., Li R. A random comparative study of terbinafine versus griseofulvin in patients with tinea capitis in Western China. Mycopathologia. 2011 Nov. Vol. 172(5). R. 365–372. 13. Gianni C. Update on antifungal therapy with terbinafine // G Ital Dermatol Venereol. 2010 Jun. Vol. 145(3). R. 415–424. 14. Kikuchi I., Tanuma H., Morimoto K., Kawana S. Usefulness and pharmacokinetic study of oral terbinafine for hyperkeratotic-type tinea pedis // Mycoses. 2008 Nov. Vol. 51(6). R. 523–531. 15. Korting HC, Kiencke P., Nelles S., Rychlik R. Comparable efficacy and safety of various topical formulations of terbinafine in tinea pedis irrespective of the treatment regimen: results of a meta-analysis // Am J Clin Dermatol. 2007. Vol. 8 (6). R. 357–364. 16. Revankar SG, Nailor MD, Sobel JD Use of terbinafine in rare and refractory mycoses // Future Microbiol. 2008 Feb. Vol. 3(1). R. 9–17. 17. Schafer–Korting M., Schoellmann C., Korting HC Fungicidal activity plus reservoir effect allow short treatment courses with terbinafine in tinea pedis // Skin Pharmacol Physiol. 2008. Vol. 21(4). R. 203–210. 18. Schmid–Wendtner MH, Korting H. Topical terbinafine. Reduction of duration of therapy for tinea pedis // Hautarzt. 2008 Dec. Vol. 59 (12). R. 986–991. 19. Singal A., Khanna D. Onychomycosis: Diagnosis and management // Indian J Dermatol Venereol Leprol. 2011 Nov–Dec. Vol. 77(6). R. 659–672. 20. Stock I. Antimycotic therapy of Tinea pedis and other foot mycoses // Med Monatsschr Pharm. 2008 Jul. Vol. 31(7). R. 247–258. Van Duyn Graham L., Elewski BE. Recent updates in oral terbinafine: its use in onychomycosis and tinea capitis in the US // Mycoses. 2011 Nov. Vol. 54 (6). R. 679–685.