Peculiarities
- Actovegin ointment belongs to a group of drugs that accelerate tissue regeneration processes. It is a white substance with a uniform consistency. The product is supplied in aluminum tubes of various sizes.
- The active component in Actovegin ointment is a deproteinized hemoderivative. This is a natural component that is obtained from the blood of calves during dialysis and ultrafiltration. The natural substance contains more than two hundred biologically active substances. In this regard, it is impossible to accurately describe the mechanism of action of the drug. The main substances in the composition of the hemoderivat:
- Amino acids important for the body.
- Compounds of peptides and carbohydrates.
- Nucleotides.
- Cholesterol.
- All of these components are natural to the human body, so the drug is well tolerated.
- Actovegin ointment has a pronounced antihypoxic effect. That is, when using the ointment, it is possible to compensate for the lack of oxygen in tissue cells, and, therefore, accelerate the regeneration processes. In addition, the use of the product increases the concentration of amino acids, improves blood circulation and normalizes metabolism at the cellular level.
Relevance of the problem
Chronic wounds have been a major global health problem for many years.
Many Russian and foreign authors have studied it, adopting various definitions of this concept and offering many treatment methods for this pathology [1, 3, 6, 9]. Despite the mass of scientific works on this topic and hundreds of proposed innovative treatment methods, the problem of treating chronic wounds is still acute for surgeons all over the world [2–4, 11, 12]. The annual cost of treating chronic wounds reaches $9 billion and requires a lot of time and effort from medical personnel to organize long-term adequate monitoring of this category of patients [5, 6, 8, 10, 12]. In 1996, the European Society for Tissue Repair at a special meeting adopted the definition: “a wound should be considered chronic if it does not heal within a period that is normal for wounds of this type or location.” Domestic authors have supplemented this definition and describe a chronic wound as a wound whose repair is impaired due to unfavorable background conditions. The concept of “chronic wound” is very broad and also includes trophic ulcers, chronic anal fissure and other long-term non-healing wound defects. The reasons behind the formation of chronic wounds are also very diverse. The etiological factors leading to the development of trophic ulcers have been well studied. They can be venous against the background of chronic lymphovenous insufficiency, arterial, with chronic ischemia of the lower extremities, against the background of diabetic polyneuropathy, micro- and macroangiopathy, hypertensive (Martorell syndrome), with systemic diseases (blood diseases, metabolism, collagenosis, vasculitis), neurotrophic, scar-trophic; congestive (against the background of circulatory failure), pyogenic, specific and infectious, malignant, with Lyell's syndrome, with congenital malformations of the vascular system, radiation, artificial [2, 3, 7]. The genesis of most chronic wounds is polyetiological, involving various pathogenetic mechanisms affecting cellular and tissue metabolism, microvascular endothelium [7, 9, 11]. In this regard, it is important to use therapeutic techniques using, in particular, pharmacological drugs that affect various stages of pathogenesis. The drug Actovegin, which has pleiotropic effects, has shown its therapeutic potential in patients with diabetes mellitus and chronic ischemia of the lower extremities, accompanied by ulcerative necrotic complications [1, 3, 4, 7]. Taking into account the above, the goal of our work was to evaluate the effects of the drug Actovegin in the complex treatment of chronic wounds of various etiologies.
Material and methods
Under our supervision were 118 patients aged from 52 to 80 years old, hospitalized in the department of vascular surgery and surgery for purulent complications of diabetes mellitus of City Clinical Hospital No. 81. All patients were treated due to the presence of chronic wound defects. The patients were divided according to the etiological factor into three groups, which included pathological conditions that cause the formation of 90% of long-term non-healing wound defects. Group 1 included 54 patients who suffered from long-term non-healing trophic ulcers of the lower extremities due to chronic venous insufficiency. In all patients, duplex ultrasound scanning (USD) of the veins of the lower extremities diagnosed the incompetence of the saphenous vein valves and the presence of long-term (from 2 to 48 months) non-healing wound defects in the lower and middle thirds of the legs. All patients underwent etiopathogenetic treatment: after a course of antibacterial therapy, taking into account the microbial landscape and cleaning of wound defects, crossectomy was performed in combination with Linton's operation. In the postoperative period, the patients were divided into 27 patients into two groups - the main and control groups, identical in gender, age and severity of concomitant pathology. The patients received drug therapy and local treatment in full. In addition to traditional treatment, patients of the main group received Actovegin intravenously (1200 mg once a day for 14 days) and topically in the form of a gel, ointment or cream, depending on the stage of the wound process:
- in the exudation stage, Actovegin 20% in the form of a gel was used in a thick layer, followed by the application of a compress with Actovegin 5% in the form of an ointment;
- in the proliferation stage, a bandage with Actovegin 5% in cream form was used;
- At the granulation stage, a bandage with Actovegin 5% in gel form was applied.
After discharge, patients were prescribed the drug Actovegin, 1 tablet (200 mg) 3 times a day for 30 days. In the control group, local treatment was carried out with ointment dressings (Betadine, methyluracil, Levosin).
Group 2 included 36 patients with symptoms of chronic arterial ischemia of the lower extremities, as well as long-term non-healing wound defects at various levels of the feet against the background of obliterating atherosclerosis and endarteritis. Ultrasound scanning of the arteries of the lower extremities revealed occlusions of the femoral-popliteal-tibial segment at various levels. All patients underwent correction of the main blood flow according to indications: femoral-distal bypass with autologous or synthetic prostheses, conservative hemorheological (reopolyglucin, dextran, etc.), anticoagulant (heparin, Plavix, Wessel Due F), antiplatelet (pentoxifylline, acetylsalicylic acid) full therapy. In the postoperative period, patients were divided into 18 people into the main and control groups, identical in gender, age and severity of concomitant pathology. In addition to standard treatment, patients in the main group received Actovegin intravenously 1200 mg once a day for 14 days, as well as locally in various dosage forms depending on the stage of the wound process:
- in the exudation stage, Actovegin 20% in the form of a gel was used in a thick layer, followed by the application of a compress with Actovegin 5% in the form of an ointment;
- in the proliferation stage, a bandage with Actovegin 5% in cream form was used;
- At the granulation stage, a bandage with Actovegin 5% in gel form was applied.
After discharge, Actovegin was prescribed 1 tablet (200 mg) 3 times a day for 30 days. In the control group, Actovegin was not used, and local treatment was carried out with Betadine solution, Levomekol ointment, and methyluracil ointment.
Group 3 included 28 patients who had suffered from type 2 diabetes mellitus for a long time, with clinical signs of polyneuropathy and long-term wound defects of the lower extremities, not associated with chronic arterial or venous insufficiency. These patients underwent correction of carbohydrate metabolism (insulin therapy), local treatment including necrectomy (according to indications), hemorheological and neurotropic therapy (Octolipen, Berlition), ultrasonic cavitation of wounds in regeneration mode every other day, dressings with Hydrosorb gel, Betadine solution. These patients were also divided into 14 people into the main and control groups, identical in gender, age and severity of concomitant pathology.
In the main group, in addition to standard treatment, patients received the drug Actovegin intravenously 1200 mg once a day for 14 days, as well as locally in various dosage forms depending on the stage of the wound process:
- in the exudation stage, Actovegin 20% in the form of a gel was used in a thick layer, followed by the application of a compress with Actovegin 5% in the form of an ointment;
- in the proliferation stage, a bandage with Actovegin 5% in cream form was used;
- At the granulation stage, a bandage with Actovegin 5% in gel form was applied.
After discharge, Actovegin was prescribed 1 tablet (200 mg) 3 times a day for 30 days.
In all nosological groups of patients, before and after courses of complex therapy, visual monitoring of wounds was carried out using the MEASURE system [11], and microcirculation studies were also performed using laser Doppler flowmetry. The sensor was located at the bottom of the wound defects. In addition, all patients underwent histological examination of wound biopsies throughout the entire period of hospital treatment in dynamics. Biopsy specimens were fixed in 10% neutral formalin and, according to the generally accepted method, embedded in paraffin blocks. Histological sections 4–5 μm thick made from paraffin blocks were stained with hematoxylin and eosin.
Results and its discussion
In patients of group 1, who were treated for chronic wounds caused by chronic venous insufficiency, a decrease in the area of wound defects (calculated from the linear dimensions of the wounds) in the main group was noted on days 4–5, in the control group – on days 7–9 -e postoperative period.
In both groups, on the 3rd–4th day of the postoperative period, the amount of exudate decreased from moderate to scanty, and we qualitatively assessed the exudate as serous or serous-hemorrhagic. The bottom of the wounds was represented by granulation tissue in both groups. In the main group, pain decreased significantly on days 2–3 of the postoperative period, while in the control group its intensity decreased only on days 5–6. No wound destruction was observed in both groups. Epithelization of the wound edges in the main group was observed on days 3–4, while in the control group – on days 6–7. At the time of discharge, chronic wounds had completely healed in 10 (37%) patients in the main group and in 5 (18.5%) in the control group. 3 weeks after discharge, the wounds healed in all (100%) patients in the main group, and in 15 (55.5%) patients in the control group.
In patients of group 2, who received treatment for chronic wounds caused by chronic arterial insufficiency, a decrease in the area of wound defects was noted in both groups, however, in the main group it occurred on days 4–5, and in the control group on days 7 –8th day after surgery. The amount of exudate decreased from scanty to absent in both groups on the 3rd–4th day of the postoperative period; we assessed the exudate qualitatively as serous-hemorrhagic. The bottom of the wounds was represented by granulation tissue on days 6–7 in 11 (61%) patients of the main group and 6 (33%) of the control group. In the remaining patients, the wounds were completely covered with hard and dry scab. In the main group, the pain syndrome decreased significantly on the 3rd–4th day of the postoperative period, while in the control group its intensity decreased only on the 6th–7th day. No wound destruction was observed in both groups. Epithelization of the wound edges in the main group was noted on days 4–6 and on days 8–10 in the control group. At the time of discharge, chronic wounds had completely healed in 7 (38.8%) patients in the main group and in 3 (16.6%) patients in the control group. During the first 3 weeks after discharge, wounds healed in 12 (66.6%) patients in the main group and in 8 (44.4%) patients in the control group.
In patients of the 3rd group who were treated for chronic wounds against the background of a neuropathic form of diabetic foot syndrome, a decrease in the area of wound defects in the main group was noted on days 2–3, in the control group – on days 4–5 after start of treatment. The amount of exudate decreased from moderate to scanty in the main group on days 1–2, in the control group on days 2–3, and we qualitatively assessed the exudate as serous-hemorrhagic. The bottom of the wounds was represented by granulation tissue in both groups. In the main group, pain decreased significantly on days 2–3 of the postoperative period, while in the control group its intensity decreased only on days 5–6. No wound destruction was observed in both groups. Epithelization of the wound edges in the main group was observed on days 3–4 and on days 5–6 in the control group. At the time of discharge, chronic wounds had completely healed in 12 (85%) patients in the main group and in 8 (57%) patients in the control group. During the first 3 weeks after discharge, wounds healed in all patients of the main group (100%) and in 12 (85.7%) patients in the control group.
Particular attention is paid to the dynamics of ulcer healing in the study groups as one of the most important indicators of the effectiveness of therapy. The results of the study showed that when using Actovegin for external use and intravenous infusions in combination, a month after the start of treatment, epithelization of the ulcer was observed in more than half (53%) of the patients, which is statistically significant.
When using Actovegin externally in the form of a gel, cream and ointment, the average time for cleansing wounds from purulent and necrotic masses, the appearance of granulations was on average less than the time in the control groups for 3-4 days (Table 1). A month after the start of therapy, epithelization of the ulcer defect was observed in 29 patients of the main group and 16 of the control group. In 9 patients of the main group, flaccid granulations with fibrin deposits were observed, in the control group - in 20 patients (Table 2).
The results of studying microcirculation using laser Doppler flowmetry are presented in table. 3.
Histological analysis of biopsy samples from wounds after a period of hospital treatment in all patients receiving Actovegin, compared with patients in the control groups, showed normalization of the processes of proliferation and differentiation of granulation tissue (collagen formation and neovascularization), less pronounced leukocyte infiltration, active processes of epidermization of skin lesions ( growth of the epithelial layer at the border with granulation tissue).
To illustrate the effects of complex therapy, we provide a clinical example.
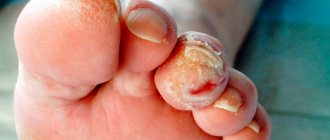
Patient V., 67 years old, with diabetic foot syndrome, neuropathy of the 2nd degree according to Wagner, trophic ulcer of the plantar surface of the resected right foot against the background of a corn for three months (Fig. 1). The patient was treated as an outpatient at the clinic; as prescribed by the surgeon, dressings with levomekol ointment were used for one month without effect. The patient was sent to the department of purulent surgery and purulent complications of diabetes mellitus as planned. During the examination: clinical and biochemical parameters were within normal limits; ultrasound scanning of the arteries of the lower extremities revealed occlusion of the distal parts of the anterior and posterior tibial arteries.
Upon admission, the patient took a biopsy from the wound (Fig. 2), normalized carbohydrate metabolism, and prescribed local treatment: ultrasonic cavitation in regeneration mode every other day, bandages with Hydrosorb gel. Along with traditional treatment, the patient was prescribed a course of Actovegin 1200 mg intravenously once a day for 14 days. On the 6th day after cleansing the wound, Actovegin gel was used in a thick layer under a bandage. During treatment, positive dynamics were noted: the size of the wound decreased, perifocal inflammation disappeared (Fig. 3). Upon discharge, the patient underwent a wound biopsy (Fig. 4). After discharge, the patient was recommended to take Actovegin on an outpatient basis, 1 tablet (200 mg) 3 times a day for 30 days, and to use Actovegin topically in gel form. On the 28th day from the start of treatment, pronounced positive dynamics were noted (Fig. 5), and on the 42nd day from the start of treatment, the patient developed a connective tissue scar at the site of the trophic ulcer of the resected right foot. The treatment result was assessed as satisfactory (Fig. 6).
In conclusion, it is worth noting that the prescription of the drug Actovegin was not accidental and is due to its pharmacological properties: pronounced metabolic and endothelial protective effects at the level of the microvascular bed. Actovegin improves collateral blood flow, trophic oxygen supply to tissues during chronic ischemia, as well as vascular and neuropathic complications of diabetes mellitus [1–3, 7]. The results obtained allowed us to draw the following conclusions and recommendations:
In the complex treatment of chronic wounds, the leading role belongs to pathogenetic treatment.
For the treatment of chronic wounds of various etiologies, the use of Actovegin as part of complex therapy reliably increases its effectiveness. When using Actovegin, the size of chronic wounds decreases 3-4 days earlier, and pain syndrome decreases 2-3 days earlier compared to patients in the control group.
The administration of Actovegin can significantly improve microcirculation in a chronic wound (the average microcirculation indicator is on average 0.2 pF higher in the group of patients receiving Actovegin).
We recommend including the drug Actovegin in the complex treatment regimen for patients with chronic wounds of various etiologies according to the following scheme: 1200 mg intravenously once a day for 14 days. Next, switch to the tablet form: 1 tablet (200 mg) 3 times a day for 30 days.
For local treatment of clean chronic wounds of various etiologies, we recommend using Actovegin in gel form once a day until complete healing.
Indications
Actovegin ointment, the price of which is affordable, is prescribed in the presence of damage to the skin in order to speed up healing. The product is indicated for the treatment of any wounds. These can be cuts, scratches, etc. It is used to treat inflammatory processes, radiation injuries on the skin and mucous membranes
Indications for use of the product are:
- Trophic ulcers that occur with varicose veins or diabetic angipatia.
- Bedsores caused by prolonged immobility of seriously ill patients.
- Burns that can occur as a result of thermal or chemical exposure, or during prolonged exposure to the sun.
Actovegin 5% 20g ointment for external use
Composition and release form Actovegin 5% 20g ointment for external use
Solution for infusion (in dextrose solution) - 250 ml active substance: deproteinized hemoderivative of calf blood - 25 ml (corresponds to 1 g of dry weight) excipients: dextrose; sodium chloride; water for injection in 250 ml bottles; 1 bottle in a cardboard pack. Solution for infusion (in sodium chloride solution 0.9%) - 250 ml active substance: deproteinized calf blood hemoderivate - 25 ml (corresponds to 1 g of dry weight) - 50 ml (corresponds to 2 g of dry weight) excipients: sodium chloride; water for injection in 250 ml bottles; 1 bottle in a cardboard pack.
Directions for use and doses
Dosage and method of use of Actovegin
depend on the nosological form and severity of the disease. 1-2 tablets are prescribed orally 3 times a day before meals. Do not chew the pills, wash them down with a small amount of water. For intravenous or intravenous administration, depending on the severity of the disease, the initial dose of the drug in the form of an injection solution is 10-20 ml. Then prescribe 5 ml IV slowly or IM 1 time/day daily or several times a week.
A solution for infusion with sodium chloride 20%, a solution for infusion with sodium chloride 10%, a solution for infusion 10% with dextrose is administered intravenously by drip or intravenous injection.
In case of disorders of blood supply and metabolism of the brain, initially 250-500 ml/day is administered intravenously for 2 weeks, then 250 ml intravenously several times a week for at least 4 weeks.
For ischemic stroke, 250-500 ml is administered intravenously daily or several times a week for about 2-3 weeks.
For arterial angiopathy, 250 ml is administered intravenously and intravenously daily or several times a week; Duration of therapy is about 4 weeks.
For trophic and other indolent ulcers and burns, 250 ml is administered intravenously daily or several times a week, depending on the speed of healing, in addition to local therapy with Actovegin
. In order to prevent and treat radiation damage to the skin and mucous membranes, an average of 250 ml is administered intravenously the day before and daily during radiation therapy, as well as for 2 weeks after its completion.
Rules for administering solutions Actovegin solutions for infusion with sodium chloride 20% and 10% and with dextrose 10% are intended for intravenous drip or intravenous jet administration.
Before starting Actovegin infusion, you must ensure the integrity of the bottle. The solution for infusion is administered in a dose of 250 ml. The initial dose can be increased to 500 ml. The infusion rate is approximately 2 ml/min. 10-20 infusions may be required to achieve the desired effect. During infusion, care must be taken to ensure that the solution does not enter extravascular tissue.
Indications for use Actovegin 5% 20g ointment for external use
- Trophic ulcers;
- bedsores;
- slow-healing wounds;
- cerebrovascular accidents;
- traumatic brain injuries;
- complications of varicose veins of the lower extremities;
- burns;
- corneal damage.
Contraindications
hypersensitivity to the drug Actovegin
.
Application of Actovegin 5% 20g ointment for external use during pregnancy and breastfeeding
It is possible to use the drug Actovegin
orally or parenterally during pregnancy and lactation as indicated.
special instructions
If allergic reactions occur, treatment is stopped. Inject slowly and not more than 5 ml intramuscularly (the solution has hypertonic properties). With repeated infusions, indicators of water and electrolyte metabolism are monitored. The infusion solution has a slightly yellowish tint, the intensity of which depends on the batch number and the starting material, but the color of the solution does not affect the effectiveness or tolerability of the drug. After opening the bottle, the solution cannot be stored. It is not permissible to use the stored solution. You cannot add any medications to the Actovegin® infusion solution due to possible pharmaceutical incompatibility. As with all other drugs intended for parenteral administration, before infusion of the Actovegin® solution, you must ensure that the vial is intact.
Contraindications and negative reactions of the body
The main contraindication for the use of Actovegin ointment, the instructions for use warn about this, is hypersensitivity to the components of the drug. Due to individual intolerance to the drug, local allergic reactions may occur. To check the body's reactions, before using the ointment, apply a small amount to the inside of the wrist. If after a couple of hours there is no redness of the skin or discomfort, Actovegin ointment can be used.
Due to the natural composition of the product, there is no categorical ban on its use during pregnancy and lactation. But at the same time, you should use the ointment only if there are indications and recommendations from a doctor.
In practice, Actovegin ointment was well tolerated. Only with hypersensitivity are local allergic reactions possible. In this case, the use of ointment should be abandoned. To eliminate side effects, it is recommended to take antihistamines.