Services Doctors Results Reviews
Expert tested
Dermatovenerologist, cosmetologist. Candidate of Medical Sciences
Publication date: May 22, 2022
Review date: November 13, 2022
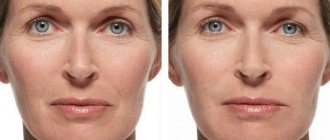
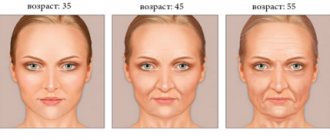
The new generation filler from the German concern “Merz” Radiesse is a real face sculptor. What many Hollywood celebrities achieved with facial implants 20-30 years ago can now be achieved with Radiesse.
The essence of the Radiesse lifting technique
Today it is generally accepted that facial modeling is carried out using 2D and 3D techniques. Do not be confused by these numbers; all symbols have been introduced to facilitate mutual understanding between cosmetologists.
It is necessary to say about the Radiesse vector lifting procedure that it is an excellent stimulation of the body’s production of collagen. The basis of the drug is synthetic microspheres of calcium hydroxyapatite and a gel carrier. It is thanks to them that the necessary lifting effect is achieved.
Warnings
- The implant should not be inserted into blood vessels. Injection into blood vessels may cause platelet aggregation, vascular occlusion, infarction, embolism, or hemolysis.
- Should not be injected into organs or other structures that may be damaged by the space created by the implant.
- Implantation should not be performed on patients taking aspirin or other medications that may interfere with the healing process.
- Should not be implanted into infected or potentially infected tissue or into open cavities as infection or extrusion may occur. Significant infection can cause damage or loss of skin over the implant. Hematomas or seromas may require surgical drainage.
- In the event of a hypersensitivity or allergic reaction, significant inflammation or infection may occur requiring removal of the implant.
- Some injectable implants have caused hardening of tissue at the injection site, migration of particles from the injection site to other parts of the body, and/or allergic or autoimmune reactions. Based on clinical use, animal studies and relevant literature, these reactions have not been observed and are not expected to occur with the Radiesse™ Injectable Implant.
- As with any implant material, possible side effects may include, but are not limited to: inflammation, infection, fistula formation, extrusion, hematoma, seroma, calcification, problematic healing, skin discoloration, and under- or over-augmentation.
- The safety and effectiveness of the product for women during pregnancy or lactation have not been established.
- The safety and effectiveness of the Radiesse™ injectable implant for use in the labial mucosa has not been established.
Radiesse vector lifting – indications and contraindications
The drug is used if the patient wishes to eliminate age-related changes in the skin, slow down involution processes, which depend on many external factors - from lifestyle to ecology. Radiesse lifting is used for:
- restoration of facial oval;
- smoothing wrinkles in the lower and middle third of the face;
- restoration of volume of cheekbones, cheeks;
- correction of nasolabial folds;
- eliminating marionette wrinkles;
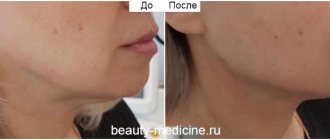
- thickening of the neck skin;
But there are also a number of contraindications.
Contraindications
inflammatory processes in the area of exposure to the drug;
allergy to the components of the drug;
tendency to hypertrophic scars;
the presence of other permanent implants in the insertion area;
systemic diseases.
On the day of the procedure, try not to drink too much coffee.
Chin correction
The ideal product for correcting the volume and shape of the chin is the new generation dermal filter Radiesse, produced by BioForm Medical in the USA.
It is a biosynthesized calcium hydroxylapatite (HAP) with the formula Ca10(PO4)6(OH)2. The synthesized form, similar to natural HA, is capable of being transformed under processing conditions into types of different density and porosity, each of which will have its own distinctive features and properties. The scope of application of the drug is not limited to aesthetic medicine. Today, Radiesse is widely used in urology, for example to treat vesicoureteral reflux, or in surgery for procedures to restore the size of the vocal cords.
A two-phase filler consists of a carrier gel (70%) and calcium hydroxylapatite microparticles. The gel contains glycerin and water, which provides the material with excellent penetrating ability. It can be evenly distributed even in the deep layers of the dermis. The effectiveness of the drug lies in the active synthesis of collagen around the inserted implant, which is formed in the connective tissue 1.5-2 months after the injection and provides a brilliant result for 18-24 months.
It is also worth noting the high level of safety of Radiesse for the human body, which is achieved by the absence of components of animal or plant origin in the composition of the drug. This eliminates the occurrence of allergic reactions. Therefore, there is no need for pre-testing before the procedure.
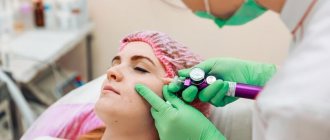
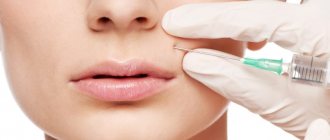
How is chin correction performed?
First, a 1-2% lidocaine solution is used as anesthesia. Radiesse is then injected from both sides into the problem area with 27 G needles. A fan technique is used. The volume of substance for correction, for example, mental folds, ranges from 0.3 ml to 0.5 ml. To correct the size of the chin, a larger amount of filler is required, the volume of which will already be from 0.5 ml to 1 ml. After the filler is injected, it is necessary to massage the area. To increase the effect, it is possible to combine injections of Radiesse and botulinum toxin type A (Botox).
Contraindications:
- pregnancy and breastfeeding;
- chronic diseases in the acute stage;
- calcification;
- violation of the blood clotting process.
It is also not recommended to perform the procedure on patients with individual intolerance to the components of the drug.
Additional information about indications for Radiesse injections:
- Correction of facial volumes
- Correction of the nasal bridge
- Hand rejuvenation
- Increasing the volume of the cheekbones
Vector lifting Radiesse - procedure diagram
Cosmetologist's comment
Oksana Grigorievna (@kosmetolog_krd)
Dermatocosmetologist
Ask a Question
To put it simply, Radiesse is a cosmetic preparation for non-surgical face lifting and long-term correction of deep wrinkles, and Radiesse vector lifting is a technique for administering the drug along Langer’s markings - the line on the face and body of maximum tension.
Regardless of the correction zone, the drug, with the vector injection technique, is injected according to the following scheme:
- after makeup removal, the cosmetologist marks the lines (vectors) along which he will work with a cannula, injecting the required dose of the drug intradermally;
- preliminary puncture sites are anesthetized with lidocaine.
The correct injection technique gives a natural lifting effect without distorting the natural contours and oval of the face.
You need to know that Radiesse vector lifting of the middle third of the face, temporal lifting, Radiesse vector reinforcement and other procedures involving the introduction of Radiesse are carried out by a cosmetologist with a higher medical education, who has been trained and received a certificate for the right to use the drug in his practice.
Correction of cheekbones with Radiesse filler
Beautiful high cheekbones give a youthful appearance. Today, it is only possible to “remove” such cheekbones without surgery using Radiesse.
Contour plastic surgery of the cheekbones with Radiesse gives a very good and long-lasting result, since the filler has a high viscosity, which is ideal for correcting this particular area. With just a few injections, the filler allows you to correct your cheekbones. This technique is characterized by the absence of a postoperative period and almost instant results.
With the help of Radiesse, new contours are formed not only of the cheekbones, but also of the cheeks, while taking into account the smallest anatomical features of the face.
It is noteworthy that Radiesse injections make it possible not only to enlarge, but also to make low cheekbones higher, and to turn thin and plump faces into normal ones.
Radiesse vector lifting – recommendations before and after the procedure
No special preparation is required for the procedure. Perhaps a specialist will recommend a vascular stage, but not always. Before visiting a cosmetologist, give up alcohol 3-4 days in advance; if you have any contraindications from the list above, be sure to inform your specialist about them.
After the procedure, there may be slight swelling and redness at the sites where the cannula was inserted. To speed up the process of recovery and regeneration, follow the following recommendations for 10 days:
- do not visit baths, saunas, swimming pools;
- exclude solariums and maximize sun exposure, use SPF +30 cream
- do not apply cosmetics (foundation, etc.);
- exclude peelings and scrubs;
- exclude alcohol.
This is a basic list; after the procedure, the cosmetologist will give additional recommendations individually.
Combination with hardware procedures
Unlike most fillers, Radiesse goes well with hardware cosmetology techniques. 3-4 weeks after administration, procedures such as microcurrent therapy, radiofrequency lifting and even hardware massage can be used. However, in most cases, hardware techniques are recommended to be used before injection procedures.
It is not recommended to administer this drug in combination with thread lifting or any type of filler, be it hyaluronic acid filler or a history of non-absorbable filler. In case of a long-term multi-component rejuvenating program, it is better to leave the introduction of Radiesse for the final stage, 2 weeks after active cosmetic interventions.
Radiesse vector lifting price by zone
| Services | Price |
| Vector lifting of the middle third of the face Radiesse - 3 ml. | 30000 |
| Vector lifting Radiesse neck - 1.5 ml. | 20000 |
| Radiesse vector lifting – 0.8 ml. | 12000 |
| * Discounts apply: | |
| — 10% for the second syringe as part of one procedure. | |
| — 15% for the third syringe as part of one procedure. | |
| * When you sign up online on the website, you receive a 5% discount on any procedure!!! | |
Individualization of therapy
Before the procedure, the patient's suitability for therapy should be assessed, as well as the patient's need for anesthesia. The outcome of therapy depends on the patient. In some cases, additional therapy may be required, depending on the size of the defect and the patient's needs. Additional injections may be given, but only after sufficient time to evaluate the patient's condition. Repeated injections should not be performed earlier than seven days after previous therapy.
RULES FOR STORAGE AND USE
Supply
The Radiesse™ Injectable Implant is supplied sterile and pyrogen-free in a syringe packaged in a foil pouch and box for easy storage. Each kit consists of one filled syringe containing 1.5 cc, 0.8 cc, or 0.3 cc of Radiesse™ injectable implant. The degree of accuracy of calibration of syringe divisions is ±0.025 cm3. Do not use the product if the packaging and/or syringe is damaged or if the tip or plunger of the syringe is affected or dislodged.
The contents of the syringe are intended for one patient, for single use only and cannot be re-sterilized. Repeated use may impair the functionality of the device and cause the device to malfunction. Reuse may also create a risk of contamination of the device and lead to patient infection or cross-transfer of infection, including, but not limited to, transmission of infectious diseases and transfer of blood between patients. All this, in turn, can lead to harm to the health, illness and death of the patient.
Storage
The package of Radiesse™ Injectable Implant should be stored at controlled room temperature between 15°C and 32°C. Do not use after the expiration date. Expiration dates are indicated on product labels.
Disposal
Used and partially used syringes and injection needles may be biohazardous and should be treated and disposed of in accordance with hospital procedures and local, state or federal regulations.
Guarantee
BioForm Medical, Inc. warrants that safety precautions were used in the development and manufacture of this product.
THIS WARRANTY IS IN LIEU OF AND EXCLUDES ALL OTHER WARRANTIES NOT EXPRESSLY STATED HEREIN, WHETHER EXPRESS OR IMPLIED BY OPERATION OF LAW OR OTHERWISE, INCLUDING BUT NOT LIMITED TO ANY IMPLIED WARRANTIES OF MERCHANTABILITY QUALITY AND FITNESS FOR A PARTICULAR PURPOSE.
Storage and handling of this product, as well as factors related to the patient, diagnosis, treatment, surgical procedures and other factors beyond the control of BioForm Medical, Inc., will directly affect the product and its results. This BioForm Medical, Icl. warranty is limited to product replacement; also BioForm Medical, Inc. shall not be liable for any incidental or consequential loss, damage or expense, direct or indirect, arising from the use of this product. BioForm Medical, Inc. does not extend its liability in relation to this product to other persons or official representatives.