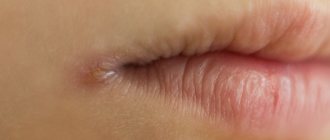
Clinical manifestations
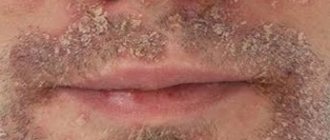
The disease can be determined by consulting a dermatologist by detecting dark brown or black spots, fibromas or papillomas, as well as signs of hyperkeratosis. The latter develops in response to exfoliation and roughening of the skin. The diagnosis requires clarification, especially since the disease is quite rare: with similar symptoms, it can easily be confused with other dermatological problems, wasting valuable time.
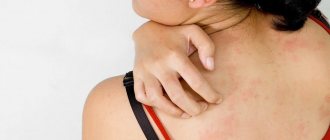
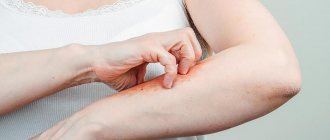
Manifestations are most often localized in the inguinal-femoral fold, on the elbows, on the skin of the armpits, in the intergluteal and popliteal areas, under the mammary glands. First, the surface darkens, then becomes rough, dry and thickens. The natural pattern takes on more pronounced features. At a later stage, the patient can seek help to remove papillomas in intimate places, however, if these formations are accompanied by tingling and mild itching, it is worth consulting with an experienced dermatologist first and undergoing additional tests.
Diagnosis and treatment
If the skin in certain areas has darkened and remains so for a long time, do not postpone a visit to the clinic. The specialist will perform a differential diagnosis, conduct a histological examination, and then confirm or refute the diagnosis.
Treatment is complex and involves both symptomatic measures and treatment of the underlying disease that provoked acanthosis. The patient may be prescribed vitamin complexes and restorative medications, anti-inflammatory ointments, zinc tablets, and baths with potassium permanganate. In severe cases, hormonal agents and cytostatics, antibacterial therapy and neurotropic drugs are used - always while following a strict diet. If the papillomas have grown seriously, they are removed.
If you discover darkening, do not put off your visit to the dermatologist. Sometimes this manifestation hides oncological diseases, which can still be affected at an early stage, while there are no general symptoms.
Why does human skin color change?
The diversity of human appearance and form has intrigued biologists and beauticians for centuries, but nearly 100 years after William Bateson coined the term "genetics" in 1906, the genes underlying this diversity remain an unsolved mystery. One of the most obvious phenotypes that distinguish our species is differences in skin pigmentation. They are among the most mysterious.
There is a huge range of human skin colors, these may be related to climate, continents or cultures, but we know very little about the underlying genetic architecture. Is the number of common skin color genes closer to 5, 50, or 500?
Do gains or losses of function for a small set of genes lead to phenotypes at opposite ends of the pigment spectrum? Did natural selection influence similar pigmentation phenotypes independently of each other and along similar pathways? And should we finally care about the genetics of a person's pigmentation if it's just skin?
Why should we take care of our skin?
From a clinical point of view, insufficient protection from sunlight has a serious impact on human health. In Australia, the cumulative lifetime incidence of skin cancer approaches 50%, but the oxymoronic "smart tanning" industry continues to grow and there are conflicting opinions about the extent to which different types of melanin influence susceptibility to ultraviolet (UV) radiation.
At the other end of the spectrum, inadequate exposure to sunlight leads to vitamin D deficiency and rickets. It was cured thanks to advances in nutrition made in the early 19th century. In both cases, understanding the genetic architecture of human skin color will likely provide greater insight into the underlying biological mechanisms, internal organs, and blood vessels. And it may also be that mutational hotspots in the TP53 gene helped educate the public about the risks of tobacco.
From a basic science perspective, changes in human skin color provide an unprecedented opportunity for cell biologists, geneticists, and anthropologists to learn more about the biogenesis and movement of subcellular organelles. To better characterize the relationship between genotypic and phenotypic diversity, to further explore human origins and understand how recent human evolution may have been shaped by natural selection.
How does skin color change?
Historically, the measurement of human skin color has been based on subjective categories, such as "medium brown, rarely burns, tans very easily." More recently, quantitative methods based on reflectance spectrophotometry have been applied, which allow for redness caused by inflammation and increased hemoglobin. To be distinguished: from darkening caused by increased melanin content
As a factor - Melanin
Melanin itself is an organic polymer built from oxidative derivatives of tyrosine and comes in two types: a cysteine-rich red-yellow form known as pheomelanin and a less soluble black-brown form known as eumelanin. Distinguishing between pigment types in biological samples requires chemical extraction, but is worth it as we know little about common changes in human pigmentation, including switching pigment types.
Characteristic light skin type
The characteristic phenotype of fair skin, freckles, and wheaten-red hair is associated with high amounts of pheomelanin and low amounts of eumelanin and is caused by loss of function alleles in one gene, melanocortin 1 receptor (MC1R). However, MC1R variation has a significant effect on pigmentation only in populations where red hair and fair skin are common, and its main effects—stimulating eumelanin synthesis at the expense of pheomelanin synthesis or vice versa—contribute little to changes in skin reflectance between or between major ethnic groups.
Changing pigment for different skin types
More important than the ratio of melanin types is the total amount of melanin produced. In addition, the histological characteristics of different skin colors provide some clues as to the cellular mechanisms that likely lead to pigment changes. For the same area of the body, people with light and dark skin have the same number of melanocytes (there is a significant difference between different areas of the body), but pigment-containing organelles called melanosomes are larger, more numerous and more pigmented in dark compared to light skin. skin, which corresponds to individuals whose recent ancestors were from Africa, Asia or Europe.
In this view, oxidative enzymes such as tyrosinase (TYR), which catalyzes the formation of dopaquinone from tyrosine, or melanosomal membrane components such as pink-eyed dilution protein or membrane-associated transport protein (MATP) influence substrate availability . and TYR activity are logical candidates that genetic variations may contribute to the diversity of human skin color.
Equally important is what happens inside melanocytes and what happens outside. Each pigment cell actively transfers its melanosomes to approximately 40 basal keratinocytes; Ultimately, skin reflectance is determined by the number and distribution of pigment granules in keratinocytes rather than melanocytes.
In general, melanosomes in African skin are larger and more widely dispersed than in Asian or European skin. Notably, keratinocytes from dark skin cultured with melanocytes from light skin induce a melanosome distribution pattern characteristic of dark skin and vice versa. Thus, at least one component of skin color change is a gene or genes whose expression and action influence the environment of the pigment cells rather than the pigment cell itself.
The genetics of skin color
For any quantitative trait, with many contributing factors, the most important questions are: overall heritability, the number of genes that may be involved, and the best strategies for identifying those genes. For skin color, heritability in the broad sense (defined as the combined influence of genetic and nongenetic factors) is very high, provided that the most important nongenetic factor, exposure to sunlight, can be controlled.
Statements about the number of human skin color genes refer to several studies; one of the most complete is Harrison and Owen (1964). In this study, skin reflectance measurements were obtained from 70 residents of Liverpool whose parents, grandparents or both were of European ("with a large Irish component") or West African ("mainly from the coastal areas of Ghana and Nigeria") origin and who were roughly classified into "hybrid" and "reverse" groups on this basis.
An attempt to separate and analyze the variance of backcross groups resulted in minimal estimates of three to four “effective factors,” in this case independently separating genes. Aside from the keyword-minimal (Harrison and Owen's data could also be explained by 30–40 genes), one of the most interesting findings was that skin reflectance appeared to be largely additive. In other words, the average skin reflectance of the "F1 hybrid" or "inverse hybrid" groups is intermediate between their respective parent groups.
An alternative approach to considering the number of potential human pigmentation genes is based on mouse coat color genetics, one of the original models for identifying and studying gene action and interaction, for which nearly 100 different genes have been identified.
Leaving aside mouse mutations that cause white patches or predominant effects outside the pigment system, there remain no more than 15 or 20 mutations, many of which have been identified and characterized and most of which have human homologues in which null mutations cause albinism.
This brings us to the question of candidate genes for skin color, since, as with any quantitative trait, a reasonable place to start is with rare mutations known to cause extreme phenotypes, in this case Mendelian forms of albinism. The basic assumption is that if a rare null allele causes complete loss of pigment, then a set of polymorphic, i.e., more frequent alleles, with subtle effects on gene expression, will contribute to the spectrum of skin colors. The TYR, P and MATP genes discussed earlier are well known causes of albinism, the main effect of restricted pigment cells; Among them, the P gene is highly polymorphic, but the phenotypic consequences of P and gene polymorphisms are not yet known.
Regardless of the phenotype, the gene responsible for selecting for different skin colors should exhibit a population signature with a large number of alleles and rates of sequence substitution that are greater for nonsynonymous (which change an amino acid in a protein) than synonymous (which do not change any amino acid) changes. Data were collected only for MC1R, in which the most notable finding is the lack of allelic diversity in African samples, which is remarkable given that polymorphism for most genes is higher in Africa than in other geographic regions. Thus, while MC1R sequence variation does not significantly contribute to human skin color variation worldwide, functional MC1R is likely important for dark skin.
Selecting skin color
The primacy for describing the relationship between latitude and skin color in modern humans is usually attributed to the Italian geographer Renato Basutti, whose widely reproduced "skin color maps" illustrate the correlation of dark skin with equatorial proximity. Later research by physical anthropologists confirmed and expanded these observations; a recent review and analysis of data from more than 100 populations found that skin reflectance at the equator is lowest, then gradually increases, about 8% at 10° latitude in the northern hemisphere and about 4% at 10° latitude in the southern hemisphere. This pattern is inversely correlated with ultraviolet radiation levels, which are higher in the southern than in the northern hemisphere. An important caveat is that we do not know how UV exposure patterns have changed over time; More importantly, we don't know when skin color likely changed, with multiple migrations out of Africa and extensive genetic exchange over the last 500,000 years
Despite this, most anthropologists agree that differences in ultraviolet radiation caused the choice of dark skin in humans at the equator and light skin in humans at higher latitudes. This remains controversial. The most popular theory states that the protection provided by dark skin from ultraviolet radiation becomes a disadvantage in more polar latitudes due to vitamin D deficiency (Murray, 1934). UVB (short-wave UV) converts 7-dehydrocholesterol into an essential precursor of cholecaliferol (vitamin D 3)); When dietary supplementation is not otherwise provided, vitamin D deficiency causes rickets, a characteristic growth pathology and bone deformities.
An often-cited anecdote in support of the vitamin D hypothesis is that Arctic populations whose skin is relatively dark given their latitude, such as the Inuit and Sami, Chukchi and Eskimos, ate diets rich in vitamin D. The sensitivity of modern humans to vitamin D deficiency is evident from -due to the widespread prevalence of rickets in industrial Europe in the 19th century, but whether dark-skinned people migrating to the polar latitudes tens or hundreds of thousands of years ago encountered such problems remains in question! In any case, the risk of vitamin D deficiency can only be explained by choosing light skin. The most reliable protection against sunburn and skin cancer is due to the physical barrier created by epidermal melanin.
Solving the mystery of pigmentation
Recent developments in several areas present an exciting opportunity to better understand the diversity of human pigmentation. Improved spectrophotometric tools, advances in epidemiology and statistics, multiple genome sequences, and efficient methods for analyzing sequence variations give us the opportunity to replace misunderstandings and myths about skin color with a scientific approach.
The same approaches that have been used to study traits such as hypertension and obesity—genetic and association studies—can be applied in a much more powerful way to study human pigmentation, since the sources of environmental variation can be controlled and we have deeper knowledge of the basic sciences: biochemistry and cell biology.
This approach is particularly attractive given the dismal success rate in molecular identification of complex genetic diseases. In fact, knowledge of the genetic architecture of skin color may be useful in designing studies of other quantitative traits.
Current debates in the human genetics community include strategies for selecting populations and candidate genes to study the characteristics of polymorphisms that should be considered as potential disease mutations and the extent to which common diseases are caused by common (and presumably ancient) alleles. Although the specific answers will be different for each phenotype, there may be common themes and some answers are better than nothing.
Harrison and Owen concluded their study of human skin color in 1964, stating: "The shortcomings of the data in this study are highly appreciated by the authors, but since there is no opportunity at present to improve the data, it seems warranted to analyze it as far as possible." Almost 40 years later, possibilities are opening up and the mystery of human skin color may be solved.