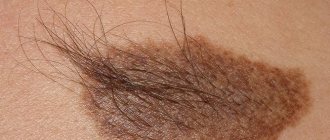
A dysplastic nevus is a pigmented formation that has characteristic clinical and histological features that distinguish it from a noncellular nevus. Dysplastic nevus, which occurs in 5-9% of the white population [1, 2], has recently attracted the attention of researchers, as it may be a precursor to superficial spreading melanoma: it is found in almost all patients with hereditary melanoma and in 30-50% patients with sporadic melanoma [1]. Dysplastic nevus can occur on intact skin or be a component of a complex noncellular nevus [1, 3].
Dysplastic nevi were first described in 1978 by W. Clark et al. [3]. When examining patients with hereditary melanoma and their relatives, the authors identified special pigmented formations that histologically consisted of atypical melanocytic hyperplasia, lymphoid infiltration, tender fibroplasia and newly formed blood vessels that occur in a mixed nevus or de
novo
. The authors explain that the term melanocytic hyperplasia is synonymous with melanocytic dysplasia, when individual melanocytes or small groups of them have some structural features of malignant melanocytes, the development trend of which is not yet clear. W. Clark et al. draw an analogy with cervical dysplasia, when foci of epithelial cells have signs of malignancy, but can remain indolent, regress or progress to overt cancer.
The term dysplastic nevus was proposed by D. Elder et al. [4], who, continuing the research of W. Clark, identified nevi with melanocytic dysplasia in patients with non-hereditary, i.e., sporadic melanoma. The authors designated this phenomenon as “dysplastic nevus syndrome.” They found two morphological types of intraepidermal melanocyte growth disorder in patients with this syndrome: the most common type, lentiginous melanocytic dysplasia (LMD), was observed in all dysplastic formations and resembled the changes occurring in lentigo simplex
, which is reflected in the name “lentiginous”. A second type of growth disorder, epithelioid cell melanocytic dysplasia, was found in two lesions in addition to lentiginous dysplasia. This pattern of growth resembled classic superficial spreading melanoma.
In 1982 Z.V. Golbert, professor at the Moscow Research Institute P.A. Herzen, regarding LMD as a variant of Dubreuil’s melanosis, similar to the stages of development of this disease, identified three degrees of development of LMD [5]. In grade I, there was an increase in the number of melanocytes in the basal layer of the epidermis and some atypia (atypia of location, increase in size, enlargement of the nucleus, hyperchromia, nuclear polymorphism were noted in individual cells). In grade II, there was a more pronounced proliferation of melanocytes, in some places completely replacing the basal row of keratinocytes, and an increase in signs of their anaplasia. This stage is characterized by the presence of elongated acanthotic cords and the tendency of melanocytes to gather in groups and clusters, especially in the acanthotic cords, which created their flask-shaped thickening. The latter structures are especially characteristic of stage III LMD: there was a tendency for melanocytes to grow into the higher layers of the epidermis, as well as for atypical melanocytes to grow deeper into the papillary layer of the dermis. This picture approximates that of melanoma in situ
.
A similar dysplastic nevus was discovered in 2005 by M. Sachdeva et al. [6]: they described a lesion that does not fit into the classic picture of a dysplastic nevus, but does not yet have sufficient evidence for melanoma in situ .
Z.V. Golbert et al. [5] noted that with LMD, as well as with Dubreuil’s melanosis, from the very beginning of the proliferation of melanocytes, even without noticeable signs of their atypia and anaplasia, there is a reactive lymphoplasma cell infiltration and angiomatosis at the base of the dermis. The similarity of the morphological picture of dysplastic nevus and lentigo maligna (Dubrey's melanosis) was also revealed by F. Farrarhi et al. [7].
Clinically, dysplastic nevus is similar to borderline nevus, but there are also differences [1, 2, 8-10]. Thus, a dysplastic nevus is an irregularly shaped spot, while a borderline nevus has a regular shape - round or oval. The color of a dysplastic nevus is often heterogeneous, with areas of dark pigment, while a borderline nevus is characterized by a uniform color, the color of both nevi varies from light brown to dark brown. Often, a punctate formation resembling a target is noted in the center of a dysplastic nevus [9]. When dysplastic and mixed nevi are combined, there is a picture of “fried eggs” with a raised yolk in the center [2]. Noncellular nevi appear in childhood or adolescence, go through stages of development from borderline to mixed and intradermal, and in old age they lose pigment and become fibrotic. Dysplastic nevi appear throughout life, from adolescence to old age, remain dormant for a long time or disappear, and fibrosis is not typical for them [1, 2]. A. Mindy [2] reports that 20% of patients with dysplastic nevi over 50 years of age noted the appearance of new pigmented formations in adulthood.
The final diagnosis of dysplastic nevus is established by histological examination. Due to certain difficulties in the differential diagnosis of noncellular nevus and LMD, the study should be carried out by a pathologist with experience in diagnosing melanocytic formations.
In our observations, dysplastic nevi were found in 5-10% of patients who consulted a surgeon or oncologist for various diseases. In some, the nevi were single (from 3 to 10 formations), in others they were multiple (from 50 to 100 or more). Observing patients with multiple dysplastic nevi, we identified two types of these formations.
In the first type (there were fewer such patients), dysplastic nevi appeared in childhood and adolescence and were often hereditary, but these patients did not report melanoma in relatives. Carriers of nevi were, as a rule, white-skinned, poorly tanned, with blond or red hair and light eyes. Dysplastic nevi of this type were large (0.5-1.0 cm in diameter), located on open and closed areas of the body (buttocks, lower abdomen), and were often combined with papillomatous nevi. The color of nevi in the same patient could vary from pink to dark brown, sometimes a variegated color was observed: dark areas on a pink or light brown background. A similar picture of nevi was described by W. Clark et al. [3] as B-K
mole
syndrome and D. Elder et al. [4] as “dysplastic nevus syndrome”. After puberty, new nevi did not appear in this group of patients. This fact can be attributed to the fact that white-skinned people limited their exposure to the sun, as they quickly burned, and were also informed about the possible appearance of new “moles” as a result of exposure to ultraviolet radiation.
In the second type, dysplastic nevi in adolescence were rare; most nevi appeared in adulthood and were associated with frequent and prolonged exposure to the sun while vacationing in southern latitudes. Dysplastic nevi of this type were small (from 0.1 to 0.4 cm in diameter), regular rounded in shape, uniform in color, combined with multiple freckles in young patients and pigment spots such as Dubreuil’s melanosis in older patients. In white-skinned patients with skin phototype I-II, the nevi were light brown, in others they were brown or dark brown. The density of nevi was higher on sun-exposed areas of the body: on the face, forearms, outer surface of the shoulders, upper back and chest wall (like a “wide neckline”). M. Sachdeva et al. [6], observing 75 patients with dysplastic nevi that arose de
novo
, also noted that in 73 patients the nevi were located on sun-exposed areas of the body. All this indicates some similarity between dysplastic nevi of the second type and Dubreuil’s melanosis, which is a proliferation of melanocytes in the basal layer of the epidermis in individuals with skin phototype I-II under the influence of repeated sunburn.
Material and methods
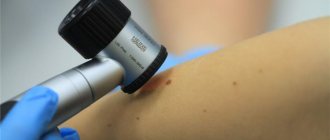
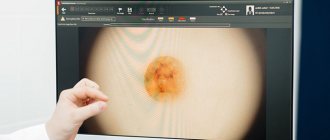
From June 2009 to April 2014, in the surgical department of the Central Clinic of the Literary Fund, for the purpose of prevention and early diagnosis of skin melanoma, 82 pigmented formations with a clinical diagnosis of dysplastic nevus were excised. The diagnosis was made based on clinical signs: irregular nevus shape, very dark or uneven color. By collecting anamnesis, the researchers tried to find out whether the nevus had existed since childhood or appeared in adulthood, and whether there had been any changes in it in the last 1-5 years. Inspection of nevi was carried out in good lighting; a dermatoscope was used if necessary to differentiate a nevus from non-melanocytic skin formations (keratomas, hemangiomas, dermatofibromas, etc.). It should be noted that recently the possibilities of dermatoscopy have expanded and with some experience it can help in the differential diagnosis of benign nevus and early melanoma, therefore dermatoscopy should be in the arsenal of a dermatologist when examining melanocytic formations.
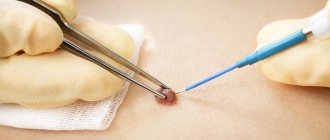
Excision of dysplastic nevi with subcutaneous tissue was carried out on an outpatient basis under local anesthesia, at a distance of 0.4-1.0 cm from the visible boundaries. Histological examination was carried out in the Clinical Diagnostic Department of OJSC "Medicine", if necessary, the preparations were reviewed at the Moscow Research Institute of Orthopedics. P.A. Herzen.
Often patients refrained from excision of the nevus, explaining that it did not bother them. In these cases, we scheduled a repeat examination after 6 months. If changes in the nevus were detected over the elapsed period, surgery was re-offered; if there were no changes, further observation was continued. We recommended that all patients with dysplastic nevi avoid prolonged sun exposure, protect their skin from the sun with clothing, and use sunscreen.
It should be noted that almost all dysplastic nevi in our observations were discovered during a routine examination by a surgeon, oncologist or dermatologist. The patients themselves did not consult doctors about dysplastic nevi, since they did not bother them. Therapists also rarely referred patients with dysplastic nevi to an oncologist. All this can be explained by the fact that both patients and doctors believe that all flat pigmented formations are benign and do not pay attention to them. Foreign authors also note that doctors and patients have a misconception about dangerous pigmented formations [11]. It is necessary to ensure that doctors and patients pay attention not to papillomatous nevi, which are benign, but to flat pigmented formations that have an unusual shape and dark or uneven color.
Causes
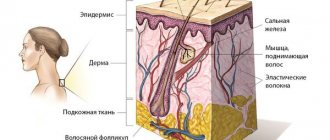
Melanocytes (cells that synthesize melanin) are located in the layers of the epidermis. This pigment protects the skin from the negative effects of ultraviolet radiation.
What happens if you scratch a mole
Moles (nevi) are formations that appear on the human body in childhood.
Under normal conditions, melanin is evenly distributed throughout the layers of the epidermis. But in people with a genetic predisposition to the formation of moles, melanocytes are concentrated in certain areas, which contributes to the appearance of Clark nevi.
The process of formation of age spots does not depend on human actions. The area in which moles form is determined at the stage of fetal development. This explains the reason why nevi occur in children in the first years of life.
In the future, the appearance of pigment spots is caused by the influence of external or internal factors. The first is ultraviolet radiation.
Prolonged exposure to sunlight triggers mutations responsible for the appearance of nevi. In some people, age spots occur due to the activity of certain viruses (in particular, the human papillomavirus). The influence of this factor stimulates the division of melanocytes.
In addition, the likelihood of nevus formation is directly related to hormonal status. Women often develop new birthmarks during pregnancy. Some relationship has also been identified between the state of immunity and the appearance of nevi.
results
Of the 82 removed pigmented formations, the clinical diagnosis of dysplastic nevus was confirmed morphologically in 62 cases, i.e., LMD structures were found in these formations during histological examination. In 2 cases, melanoma was detected that developed against the background of LMD, in 7 - intradermal nevus, in 10 - mixed nevus, in one - nevus of the sebaceous glands.
Morphologically verified dysplastic nevi and two melanomas were removed from 45 patients (in some individuals, 2-3 nevi). The distribution of patients by age was as follows: 20-29 years - 11 people, 30-39 years - 18, 40-49 years - 9, 50-59 years - 1, 60-69 years - 6. Melanoma was identified in women 49 and 30 years. In total there were 36 women, 9 men. The population of the clinic consists mainly of young and mature people, most of them are female, which was reflected in the gender and age composition of the patients. Some authors [10], however, note that melanoma is the most common malignant tumor in women 25-29 years old.
The removed dysplastic nevi were small in size: 0.3 cm in 3 cases, 0.4 cm in 11 cases, 0.5 cm in 10 cases, 0.6 cm in 5 cases, 0.7 cm in 13 cases, 0.8 cm - in 4, 1.0–1.5 cm in diameter - in 16 cases. Melanoma in 1 case had dimensions of 0.5×0.4 cm, in another - 0.5 cm. As can be seen from the data presented, dysplastic nevi 0.5 cm or less were noted in 24 cases. Foreign authors [1, 2, 10] recommend removing dysplastic nevi measuring 0.6 cm or more; according to our studies, smaller nevi (0.4–0.5 cm in diameter) should also be removed.
Most of the removed dysplastic nevi were localized on the trunk, of which 21 were on the abdominal wall, 7 in the upper back, 7 in the lumbar region. Fewer nevi were located on the chest wall - 4, below the shoulder blades - 1, in the sacrum - 2. There were two nevi in the groin area, 10 on the lower limb, 7 on the upper limb, and 1 on the neck. The melanoma was localized on the forearm and chest wall.
Considering the distribution of dysplastic nevi on the body, it can be assumed that on vacation in hot countries, the upper back, chest, abdominal wall and lumbar region are exposed to intense solar radiation.
In a histological study of 62 dysplastic nevi, LMD that developed de
novo
was found in 24 cases; in 38 cases, there was a combination of LMD with a mixed, intradermal or borderline nevus (see table). Our studies are consistent with the data of other authors, who note that dysplastic nevi in the majority are complex nevi with components of a borderline and intradermal nevus [5, 6, 9]. In two of our cases, melanoma was also detected against the background of a mixed nevus.
The degree of development of lentiginous melanocytic dysplasia identified during histological examination of removed dysplastic nevi.
As can be seen from the table, LMD of II-III degree was identified in 20 formations, i.e. in these cases there was a progressive dysplastic nevus.
Analyzing the clinical signs of 20 progressive dysplastic nevi (with histological signs of stage II-III LMD) and two melanomas, we identified some features that distinguish them from nevi with mild (I and I-II) degree of dysplasia.
According to our observations, the most important sign of the progression of a dysplastic nevus is the appearance of a pigmented formation on unchanged skin and its further growth over several months or years in people over 18 years of age, i.e. in adulthood. Changes in the last 3-5 years of a long-existing nevus may indicate the progression of a dysplastic nevus, which is combined with a borderline or mixed nevus.
An important sign of a progressive dysplastic nevus is a very dark color (almost black) or uneven coloration of the formation with areas of dark brown or black. The irregular shape of the nevus may be little noticeable with a small size of the progressive dysplastic nevus, but at the same time it can also be observed in a long-existing noncellular nevus - borderline or mixed.
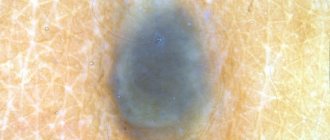
In Fig. Figure 1 shows a dysplastic nevus without signs of progression, 0.6×0.5 cm in size, light brown in color, with a characteristic “target” in the center. According to histological examination, stage I-II LMD.
Rice. 1. Dysplastic nevus without signs of progression. According to histological examination, stage I-II LMD.
In Fig. Figure 2 shows a dysplastic nevus measuring 1.0×0.4 cm, also without signs of progression: oval in shape, with smooth contours, dark brown in color. According to histological examination - mixed pigmented nevus with foci of stage I LMD.
Rice. 2. Dysplastic nevus without signs of progression. According to histological examination - mixed pigmented nevus with foci of stage I LMD.
In Fig. Figure 3 shows a nevus of the chest wall in a 27-year-old patient, 0.3 cm in diameter, round in shape, which darkened after staying in the south (a sign of progression). According to histological examination, mixed dysplastic pigmented nevus with severe LMD (grade III).
Rice. 3. Dysplastic nevus with signs of progression. According to histological examination - mixed dysplastic pigmented nevus with severe melanocytic lentiginous dysplasia (grade III).
In Fig. Figure 4 shows a nevus of the gluteal region measuring 0.5×0.4 cm in a 29-year-old patient with skin phototype I. The nevus, according to the patient, appeared 7 months ago in the form of a punctate formation, which gradually increased in size (a sign of progression). No other pigmented nevi or freckles were found on the body. According to histological examination, lentiginous pigmented nevus with severe LMD (grade III).
Rice. 4. Dysplastic nevus with signs of progression.
Progressive dysplastic nevi require excision for the purpose of prevention, as well as early diagnosis of skin melanoma. When melanoma is detected, the question of the need for reoperation (excision of the postoperative scar) is decided depending on the thickness of the tumor, determined by histological examination. According to foreign authors, as well as WHO recommendations, an adequate deviation from the boundaries of the formation for melanoma in
situ
is 0.2-0.5 cm, for invasive melanoma with a thickness of less than 1.5 mm - 1.0 cm [12].
In our observations, in a case where melanoma in
situ
, the formation was excised at a distance of 0.4 cm, so reoperation was not required. The patient has been observed for 5 years without recurrence of the disease. In the second case, in a 30-year-old patient with a clinical diagnosis of dysplastic nevus of the chest wall, the formation was excised at a distance of 1.0 cm from the visible boundaries. Histological examination revealed melanoma with a thickness of less than 0.75 mm, Clark invasion level - 2, reoperation was also not possible required.
About Clark's nevus
Clark's dysplastic nevus is a benign formation (nevoid tumor) that occurs on the body of white-skinned people. Similar spots are diagnosed in 5% of the world's population. The size of a dysplastic nevus varies between 5-15 cm.
The pigment spot does not rise above the skin. Most patients develop complex nevi, divided into 2 parts. In the center of such nevoid tumors there is a benign neoplasm surrounded by melanocytes. The presence of the latter explains the absence of clearly defined boundaries in nevi.
The first age spots appear in childhood. As a person gets older, the number of Clark's nevi usually increases. A nevoid tumor consists of immature cells that produce melanin.
This mechanism, according to the researchers, determines the relationship between exposure to sunlight and cell mutation. Frequent and prolonged contact of ultraviolet radiation with nevi contributes to the degeneration of the latter into cancerous tumors.
conclusions
1. Pigmented nevi of irregular shape, dark or uneven color should be detected during a preventive examination of the skin by therapists and surgeons. Patients with such nevi should be referred to dermatologists, whose task is to identify dysplastic nevi with signs of progression and refer patients for excisional biopsy.
2. The most significant sign of a progressive dysplastic nevus is the appearance of a pigmented formation on unchanged skin and its growth in persons over 17 years of age, as well as changes in the existing nevus over several years or months in persons after puberty. Important signs of progression of a dysplastic nevus are very dark or uneven coloration with areas of dark brown or black.
3. Excision of the nevus (excisional biopsy) should be performed with subcutaneous tissue under local anesthesia, departing from the visible boundaries of 0.4-1.0 cm. Histological examination of the removed nevus should be carried out by a pathologist with experience in studying melanocytic formations.
4. If the patient refuses surgery or in the absence of obvious signs of progression of the dysplastic nevus, a re-examination should be scheduled after 6 months. If there are changes in the nevus over the past period, surgery is suggested; if there are no changes, further observation is recommended. Patients with dysplastic nevi should be advised to avoid prolonged exposure to direct sunlight, protect the skin with clothing, and use sunscreen.
Clinical manifestations
In most cases, it is not difficult to determine the specific type of nevoid skin formations after a detailed examination of them.
The following features of their appearance may suggest the idea of dysplastic nevi:
- They acquire their atypical appearance from the moment of their appearance;
- They can be either isolated or spread over the entire surface of the skin;
- Most actively appear in puberty and young people
The appearance of dysplastic nevi is presented in the table:
| Quantity | From 1-2 to 100 or more |
| Favorite localization | Mainly arms and upper torso |
| Dimensions | Always large (about a centimeter) |
| Coloring | Contains pigment elements with colors of varying intensity (from light light pigmentation to rich brown). Characteristic is the alternation of areas of different intensity within one nevus |
| Elevation above the surface of the skin | Protrudes slightly above the surface of the skin or is at its level |
| Surface | It's never smooth. It is characterized by a finely tuberous structure, reinforced in the central sections, especially with elevated types of nevi. Peripheral sections are less elevated and smooth |
| Form | Most often irregular, edges uneven and fuzzy |
| Additional signs | Very often there is a growth of pathological hairs from the nevus (black, long, thick) |
Histology of the removed mole
The process of malignancy can affect small moles, and when performing digital dermatoscopy, external changes in the nevus may be absent. In this regard, an erroneous treatment tactic may be undertaken to radically remove the mole using a laser, cryodestruction or electrocoagulation. In this case, it is impossible to submit the mole for histology, since the nevus tissue is burned out during the procedure. In this case, a tactical error (performing an operation without histology) can have very serious consequences.
Histology of a mole after its removal is an indispensable condition of modern oncology. Based on the results of nevus histology, one can judge the effectiveness and radicality of the chosen method. Surgical protocols for melanomas and questionable moles categorically exclude the use of techniques in which nevus histology is impossible.
“Acute” surgical excision of any suspicious area of changed skin using a scalpel, followed by examination of the resected material, is the gold standard of modern oncological surgery. Unfortunately, in Russia and the CIS countries there are no strict protocols and “imperative” instructions prohibiting the use of auxiliary techniques (laser, electrocoagulation, radioknife, etc.) “at the discretion” of the surgeon, as a result of which the likelihood of error increases, and histology of the removed mole is carried out not 100% of the time.
Immunohistochemistry of melanoma
An effective diagnostic method that allows one to determine the properties of a tumor, as well as its sensitivity to certain types of antitumor drugs, is immunohistochemistry. More than half of cutaneous melanomas have mutations in the BRAF and NRAS genes. Thanks to immunohistochemical analysis, it is possible to identify specific mutations in the nucleus of tumor cells, which makes it possible to assess the degree of effectiveness of certain target drugs and prescribe the most accurate therapy.
The method is also effective for the morphological diagnosis of anonymous metastatic neoplasms.
Specialists at the Israeli Oncology Center have extensive experience in histological diagnosis. The diagnosis of melanoma in Russia and the CIS countries differs from the diagnostic protocols used in Israel. The lack of clinical experience and the lack of high-tech equipment in most medical institutions in the CIS leads to cases of discrepancies in diagnoses and stages in the expert opinions of specialists.
In Israel, if doubts arise about the morphology and stage of the tumor, other specialists are brought in for examination (second opinion). This approach allows us to reduce the likelihood of subjective error in diagnosis to zero.
Symptoms
Most Clark's nevi have a smooth surface and do not protrude above the skin. Roughness is noted in the central part of the pigment spot. The degree of color saturation is determined by the localization of the tumor.
Why can our articles be trusted?
We make health information clear, accessible and relevant.
- All articles are checked by practicing doctors.
- We take scientific literature and the latest research as a basis.
- We publish detailed articles that answer all questions.
You can distinguish a nevus from other similar spots by the absence of clear boundaries. In addition, neoplasms of this type usually reach large sizes. If a pigment spot appears in the hair growth area, the latter acquires a dark shade.
Clark's nevi go through three stages during development. First, a chain of altered melanocytes is formed in the problem area. Then, in a limited area, the production of melanin increases, which causes the skin to darken. As the described process progresses, a bridge is formed between individual pigment spots.
The risk of developing melanoma increases from the first to the third stage. The fact that a mole has begun to mutate is indicated by the appearance of discomfort (itching, burning, pain) at the location of the pigment spot.
Also, the presence of melanoma is indicated by a change in the color of the neoplasm, the appearance of a red (inflamed) border and swelling, and a sharp increase in the number of moles on the body.
Diagnostics
The following help differentiate Clark's nevus from other skin neoplasms:
- Dermatoscopy. As part of this method, the mole is examined under multiple magnification. Dermatoscopy is effective in detecting melanoma at an early stage of development.
- Indication isotope . Used for early detection of melanoma. With such a tumor, the process of phosphorus absorption by the body is accelerated.
- Echography. It is prescribed to determine the exact location and depth of germination of skin tumors.
- Thermometry. Malignant neoplasms are characterized by a rise in temperature in the area where the tumor is located. Thermometry allows us to identify such changes.
- Radiography. During this procedure, carried out using specialized equipment, it is possible to evaluate the structure of the skin tumor and identify the presence of seals.
Sometimes, for age spots on the body, a biopsy is prescribed. More often, the method is used after removal of the tumor. This is explained by the fact that during a biopsy the integrity of the nevus is disrupted, which can lead to serious complications.