Causes of genital psoriasis and risk factors
Scientists from around the world continue to put forward hypotheses regarding the causes of psoriasis, including genital psoriasis. It should be noted that none of the assumptions made has yet been 100% confirmed. However, based on a combination of factors, experts conclude that the main role in the development of this pathology is played by an autoimmune disorder, when one’s own immune cells fight not against foreign agents, but against their own cells.
IMPORTANT! Genital psoriasis is not an infectious or viral disease. This is a dermatological problem caused by autoimmune processes. Therefore, it is impossible to become infected through sexual contact, blood transfusions, or at home.
Possible provoking factors causing genital psoriasis include:
- changes at the hormonal level (the likelihood of exacerbations increases during puberty, pre- and postmenopause, as a result of taking hormonal drugs);
- state of stress, increased nervous or physical stress;
- damage to the mucous membranes or skin in the intimate area;
- decreased immunity due to exacerbation of existing chronic diseases;
- hypothermia or prolonged exposure to damp underwear;
- allergic reactions to food, intoxication due to alcohol or medications;
- change of place of residence, moving to another climate zone;
- overweight.
Biological drugs created using genetic engineering methods are monoclonal antibodies used for therapeutic purposes. In domestic medical practice, the following biological drugs are approved for medical use for the treatment of psoriasis: infliximab, adalimumab, ustekinumab, etanercept.
1. Infliximab
Infliximab is a selective TNF-a antagonist, which is a chimeric monoclonal IgG antibody that consists of 75% human and 25% mouse protein. Infliximab is indicated for the treatment of adult patients with psoriasis with severe and moderate forms of the disease in the absence of clinical effect from the use of other systemic therapies (including cyclosporine, acitretin, methotrexate and PUVA therapy) or in cases of intolerance or contraindications to their use, as well as for the treatment of active progressive psoriatic arthritis.
Doses and regimens
The drug is administered intravenously by drip for at least 2 hours at a rate of no more than 2 ml/min. under the supervision of medical personnel. For the treatment of psoriasis and psoriatic arthritis, the initial dose of infliximab is 5 mg per kg of patient weight. After the first administration, the drug is administered in the same dose after 2, then 6 weeks. and then every 8 weeks. If there is no effect within 14 weeks. (after four intravenous infusions) it is not recommended to continue treatment. During the intravenous infusion and for at least 1-2 hours after its completion, the patient must be under the supervision of a physician. During intravenous infusion of the drug, it is necessary to measure blood pressure, pulse, and respiratory rate and body temperature every 30 minutes.
Adverse reactions/safety
In clinical studies, adverse reactions were observed in approximately 60% of patients receiving infliximab and 40% of patients receiving placebo.
- Infusion reactions occur during infusions or within 1-2 hours after it. These include swelling of the pharynx/larynx, bronchospasm, chills, headache, hot flashes, nausea, shortness of breath. In clinical trials, the incidence of infusion reactions when using infliximab was about 20%, in the comparison group (placebo) - about 10%. Approximately 3% of patients were forced to discontinue treatment due to the development of infusion reactions, which were reversible in all patients (with or without drug therapy).
- Delayed hypersensitivity reactions in the form of arthralgia, myalgia, fever and rash develop in 1% of patients with psoriasis at the beginning of treatment.
- In clinical trials, when infliximab treatment was repeated 2-4 years after the previous course of therapy, patients experienced adverse reactions (myalgia, arthralgia accompanied by fever and/or rash, itching, swelling of the face, lips or hands, dysphagia, urticaria, pain sore throat and/or headache), which developed 3-12 days after re-infusion.
- Infectious complications are the most common serious side effects. They were associated with approximately 50% of all recorded deaths. There have been cases of tuberculosis, including miliary tuberculosis with extrapulmonary localization, in some cases with a fatal outcome.
- Malignant neoplasms and lymphoproliferative diseases. There have been cases of emergence or recurrence of malignant neoplasms. The incidence of lymphoma in patients treated with infliximab was higher than the expected incidence of this disease in the general population. The incidence of other forms of malignant neoplasms in patients treated with infliximab did not exceed the reported incidence in the general population.
- Cardiovascular failure. Cases of progression of cardiovascular failure during the use of infliximab have been described. There are rare reports of newly diagnosed cardiovascular failure, including in patients who had no previous diseases of the cardiovascular system.
- Changes in the liver and biliary tract. There are very rare reports of the appearance of jaundice and non-infectious hepatitis, in some cases having signs of autoimmune hepatitis, the development of liver failure, leading to the need for a liver transplant or fatal outcome. A cause-and-effect relationship between the occurrence of these adverse reactions and treatment with infliximab has not been established. There have been cases of exacerbation of hepatitis B in patients who were chronic virus carriers (who had a positive reaction to HBsAg).
- When treated with infliximab, a mild to moderate increase in aminotransferase activity may be observed without the development of significant liver damage. In most cases, the increase in aminotransferase levels is transient and asymptomatic. A decrease or return to the initial level of these indicators occurs regardless of whether treatment with infliximab is continued or stopped or concomitant therapy is changed. An increase in alanine aminotransferase activity to a level equal to or greater than 5 times the upper limit of normal was observed in 1% of patients.
- When using infliximab, isolated cases of the development of demyelinating diseases of the central nervous system, optic neuritis, and epileptic seizures have been described.
- In some patients, antinuclear antibodies appeared in the blood serum during treatment with infliximab. Cases of reversible lupus-like syndrome have been described.
Pregnancy/lactation
Infliximab is not recommended for use during pregnancy. During treatment with the drug and for 6 months. after its completion, reliable methods of contraception should be used. Breastfeeding should be discontinued when infliximab is prescribed. Breastfeeding is allowed no earlier than after 6 months. after the end of therapy.
Prevention and treatment of adverse reactions
For early detection of an acute infusion reaction, the patient should be carefully observed during and for at least 1-2 hours after the drug infusion. If an acute infusion reaction occurs, the drug should be discontinued. When conducting infliximab infusions, it is necessary to have appropriate equipment and medications (adrenaline, glucocorticosteroids for parenteral administration, antihistamines, equipment for artificial ventilation). To prevent mild and transient infusion reactions, the patient may be prescribed antihistamines before starting the infusion.
Contraindications/restrictions
- Hypersensitivity reactions to infliximab, other murine proteins, as well as to any of the inactive components of the drug.
- Severe infectious process, for example, sepsis, abscess, tuberculosis or other opportunistic infection.
- Heart failure - severe or moderate.
- Pregnancy and breastfeeding.
- Age less than 18 years.
Interaction
The interaction of infliximab with other drugs has not been studied. Concomitant use with methotrexate in patients reduces the formation of antibodies to infliximab and increases its concentration in plasma.
Overdose
A single administration of infliximab in doses up to 20 mg/kg did not cause direct toxic effects. In case of overdose, observation and immediate relief of symptoms are necessary.
special instructions
- Infliximab, when administered, can cause the development of acute allergic reactions (immediate type) and delayed allergic reactions. Acute infusion reactions may develop immediately or within several hours after administration. A patient receiving infliximab should be monitored during and for at least 1-2 hours after the drug infusion.
- Some patients may develop antibodies to infliximab, which is associated with a more frequent development of infusion reactions. In patients suffering from Crohn's disease, there has been a correlation between the formation of antibodies and a decrease in the duration of the effect of treatment. Patients who stop taking immunosuppressive drugs before or during treatment with infliximab are more at risk of developing these antibodies. If severe reactions develop, symptomatic therapy should be carried out, and further use of the drug should be excluded.
- As the interval between infusions increases, the likelihood of the formation of antibodies to infliximab increases. When re-prescribing infliximab after a long break in treatment, it is necessary to be wary of the appearance of a delayed-type hypersensitivity reaction in the patient.
- When prescribing infliximab to patients with a history of indications of malignant neoplasms, or when deciding whether to continue treatment with infliximab in patients with newly diagnosed neoplasms, special caution should be exercised.
- Before starting treatment with infliximab, the patient should be carefully examined to identify both active and latent tuberculosis. The examination should include:
- careful collection of anamnesis (presence of tuberculosis in the past and/or contact with tuberculosis patients);
- X-ray examination of the chest in two projections;
- conducting a tuberculin test;
- consultation with a phthisiatrician.
- If a tuberculous process is suspected, the use of infliximab should be discontinued until diagnosis is made and, if necessary, appropriate therapy should be administered.
- The patient should be informed that he needs to consult a doctor if the following symptoms appear during treatment with infliximab or after its completion: cough, weight loss, low-grade body temperature.
- During treatment and after its completion, the patient should be closely monitored for signs of possible infection. Since elimination of infliximab occurs within 6 months, the patient should be constantly under medical supervision during this period. Treatment with infliximab should be discontinued if the patient develops a severe infection, including tuberculosis, sepsis or pneumonia.
- The use of live vaccines during treatment with infliximab is not recommended.
- In rare cases, an autoimmune process may develop in genetically predisposed patients. In the event of persistent rash, fever, joint pain, fatigue, or the presence of DNA antibodies in the blood, treatment with infliximab should be discontinued.
- The benefits and risks of infliximab should be carefully weighed in patients with pre-existing or new-onset CNS demyelinating disease.
- Patients with moderate circulatory insufficiency should be carefully monitored. If symptoms of circulatory failure increase, infliximab should be discontinued.
- Patients with signs of liver dysfunction should be evaluated for liver disease. If jaundice occurs or alanine aminotransferase activity increases to levels greater than 5 times the upper normal value, infliximab should be discontinued and a thorough evaluation should be performed.
- Hepatitis B virus carriers should be examined before treatment with infliximab and continuously monitored during treatment in order to timely detect a possible exacerbation of the disease.
- The effectiveness and safety of treatment with infliximab in children and adolescents under the age of 18 years, inclusive, suffering from psoriatic arthritis and psoriasis have not been studied. Until convincing data are obtained, the drug should not be used in these age groups.
- There have been no specific studies on the use of infliximab in elderly people, as well as in people with liver and kidney diseases. There is limited experience indicating the safety of treatment with infliximab in patients undergoing arthroplasty.
Monitoring laboratory parameters during treatment with infliximab
| Infusion weeks | ||||
| Before | 2nd week | 6th week | Every 8 weeks | |
| General blood test1 | X | X | X | X |
| General urine analysis | X | X | X | X |
| ALT,AST | X | X | X | X |
| Pregnancy test | X | |||
Note. 1 Hemoglobin, hematocrit index, erythrocytes, leukocytes, leukocyte formula, platelets.
2. Adalimumab
The selective immunosuppressive drug adalimumab is a completely identical human monoclonal antibody that blocks the activity of TNF-a, a pro-inflammatory cytokine that plays a key role in the pathogenesis of psoriasis. Doses and regimen. For chronic plaque psoriasis, the initial dose for adult patients is 80 mg. Maintenance dose - 40 mg once every 2 weeks, starting a week after the initial dose. The drug is administered subcutaneously into the thigh or abdomen.
Overdose.
The maximum tolerated dose of adalimumab in humans has not been established. Repeated use of adalimumab in doses up to 10 mg/kg was not accompanied by toxic effects requiring dose reduction. In case of overdose, it is necessary to monitor adverse reactions and immediately begin adequate symptomatic treatment.
Drug interactions
In patients with rheumatoid arthritis receiving methotrexate, there is no need for dose adjustment of adalimumab or methotrexate. However, methotrexate with single and repeated use reduces the clearance of adalimumab by 29% and 44%, respectively. The interaction of adalimumab with other drugs other than methotrexate has not been studied in pharmacokinetic studies. In clinical studies, there were no signs of interaction of adalimumab with other basic drugs (sulfasalazine, hydrochloroquine, leflunomide and parenteral gold preparations), corticosteroids, salicylates, NSAIDs and analgesics.
Pregnancy and lactation.
Adalimumab is contraindicated during pregnancy and breastfeeding. Adequate and strictly controlled studies of the drug in pregnant women have not been conducted. Women of reproductive age should avoid conceiving while being treated with the drug. Given the risk of serious side effects in the newborn, it is advisable to stop breastfeeding or discontinue the drug, taking into account its importance for the mother.
Side effects
- According to clinical studies, approximately 15% of patients can be expected to develop injection site reactions, which are one of the most common side effects with adalimumab in controlled clinical trials.
- Infections: very often - respiratory tract infections (including upper and lower respiratory tract infections, pneumonia, sinusitis, pharyngitis, nasopharyngitis and herpes viral pneumonia); often - generalized infections (including sepsis, candidiasis and influenza), gastrointestinal infections (including viral gastroenteritis), skin and soft tissue infections (including paronychia, cellulitis, impetigo, necrotizing fasciitis and herpes zoster), ear infections, oral infections (including herpes simplex, oral herpes and dental lesions), genital infections (including vulvovaginal mycotic infection), urinary tract infections (including pyelonephritis); uncommon - opportunistic infections and tuberculosis (including coccidioidomycosis, histoplasmosis and complex of infections caused by Mycobacterium avium), neurological infections (including viral meningitis), eye infections, bacterial infections, joint infections.
- Neoplasms: often - benign neoplasms, skin cancer, except melanoma (including basal cell carcinoma and squamous cell carcinoma); uncommon - lymphoma, parenchymal neoplasms, neoplasms of the mammary gland, lungs and thyroid gland, melanoma.
- From the blood and lymphatic system: very often - leukopenia (including neutropenia and agranulocytosis), anemia; often - thrombocytopenia, leukocytosis; uncommon - idiopathic thrombocytopenic purpura; rarely - pancytopenia.
- From the immune system: often - hypersensitivity reactions, seasonal allergies.
- Metabolism: very often - increased lipid levels; often - hypokalemia, increased uric acid levels, pathological changes in sodium content, hypocalcemia, hyperglycemia, hypophosphatemia, increased potassium levels in the blood; infrequently - dehydration.
- From the nervous system: very often - headache; often - paresthesia (including hypesthesia), migraine, sciatic neuralgia, mood changes (including depression), irritability, insomnia, dizziness; infrequently - tremor; rarely - multiple sclerosis.
- From the senses: often - conjunctivitis, visual impairment; infrequently - blepharitis, eyelid edema, diplopia, deafness, ringing in the ears.
- From the cardiovascular system: often - arterial hypertension, hot flashes, hematomas, tachycardia; uncommon - arrhythmia, congestive heart failure; rarely - cardiac arrest, arterial occlusion, thrombophlebitis, aortic aneurysm.
- From the respiratory system: often - cough, asthma, dyspnea; infrequently - chronic obstructive pulmonary disease, interstitial lung diseases.
- From the digestive system: very often - nausea, vomiting, abdominal pain, increased activity of liver enzymes; often - dyspepsia, gastroesophageal reflux, dry mouth (sicca syndrome), gastrointestinal bleeding; uncommon - pancreatitis, dysphagia, facial edema, cholecystitis, cholestasis, increased bilirubin levels, hepatic steatosis.
- Dermatological reactions: very often - rash (including exfoliative); often - itching, urticaria, hemorrhages (including purpura), dermatitis and eczema, brittle nails, hyperhidrosis; Uncommon: night sweats, scars.
- From the musculoskeletal system: very often - musculoskeletal pain; often - muscle spasms; uncommon - rhabdomyolysis; rarely - systemic lupus erythematosus.
- From the urinary system: often - hematuria, renal failure; infrequently - nocturia.
- From the reproductive system: infrequently - nocturia, erectile dysfunction.
- From laboratory indicators: often - violations of indicators
- blood clotting (including increased aPTT), positive tests for autoantibodies (including antibodies to the double helix of DNA), increased LDH levels.
- Local reactions: very often - reactions at the injection site (including erythema).
- Other: often - chest pain, swelling; infrequently - inflammation, deterioration of wound healing.
special instructions
.
Adalimumab has a number of important features. In particular, the drug has no risk of infusion reactions, since adalimumab is administered subcutaneously. The frequency of formation of antibodies to adalimumab is only 1.9-8.4%, which undoubtedly has a beneficial effect on both the safety profile of long-term use of the drug and has a positive effect on the level and duration of clinical response to treatment.
Long-term follow-up of psoriasis patients treated with open-label adalimumab over 3 years in the large REVEAL study is available. The effectiveness and safety of continuous therapy with adalimumab in patients with moderate to severe psoriasis for more than three years was assessed. The studies demonstrated that in patients with clinical responses to treatment PASI 75, PASI 90 and PASI 100, reflecting a decrease in the extent and severity of the skin process by 75%, 90%, 100%, respectively, the effectiveness of adalimumab was maintained for three years. Thus, after 160 weeks of continuous treatment with this biological agent, the percentage of patients with PASI 75 remained at 76%; PASI 90 - respectively 50%; PASI 100 - 31%. The data obtained suggest that the majority of patients with psoriasis who received adalimumab responded well to treatment carried out for more than 3 years. More than half of the observed individuals after 160 weeks of treatment with adalimumab had only minimal manifestations of psoriasis on the skin, and in one third of the patients the psoriatic process resolved completely. The most stable response to long-term treatment with adalimumab was found in patients with an improvement in PASI 100. The study made it possible to significantly clarify the data on a possible decrease in the effectiveness of the biological agent with long-term use. It was found that it is extremely important to assess response early in treatment with adalimumab. A good initial response rate with adalimumab (PASI 75 or more at weeks 16 and 33) is, in fact, a predictor of favorable long-term treatment outcomes over a follow-up period of 3 years or more. Finally, the study demonstrated the high safety of long-term use of adalimumab. It is important that among all study participants (n=1159), only 2 cases of tuberculosis were registered. Not a single case of lymphoma, lupus-like syndrome, or demyelinating disease was identified.
According to an analysis of the safety of adalimumab in psoriasis, where the assessment was based on data from 13 clinical studies, and the duration of treatment with the drug was up to 5 years, the frequency (expressed as the number of events per 100 PL) of serious AEs, severe infections, malignant neoplasms does not increase or even decreases as increasing the duration of treatment.
3. Ustekinumab
Ustekinumab is a fully human IgG1k monoclonal antibody that has high affinity and specificity for the p40 subunit of human interleukins (IL) IL-12 and IL-23. Ustekinumab is indicated for the treatment of patients over 18 years of age with moderate to severe plaque psoriasis, as well as patients with active psoriatic arthritis as monotherapy or in combination with methotrexate.
Directions for use and doses
Ustekinumab is intended for subcutaneous injection. The recommended dose is 45 mg. The second injection is given 4 weeks after the first application, then every 12 weeks. In patients weighing more than 100 kg, the drug is recommended to be used at a dose of 90 mg. For patients in whom the clinical effectiveness of the drug when used every 12 weeks is not sufficiently expressed, the dose of the drug should be increased to 90 mg every 12 weeks. If this dosing regimen is not effective, a dose of 90 mg should be administered every 8 weeks. Resumption of therapy according to the proposed regimen - a second injection 4 weeks after the first use, and then every 12 weeks - was as effective as the first therapy .
Side effects
The following side effects were observed in clinical studies of ustekinumab:
- infections (odontogenic infections, upper respiratory tract infections, nasopharyngitis, inflammation of subcutaneous fat, herpes zoster, viral infections of the upper respiratory tract);
- mental disorders (depression);
- from the central nervous system (dizziness, headache);
- from the respiratory system (pain in the throat and larynx, nasal congestion);
- from the gastrointestinal tract (diarrhea, vomiting);
- from the skin and subcutaneous tissues (itching);
- from the musculoskeletal system (back pain, myalgia, arthralgia);
- general disorders and reactions at the injection site (fatigue, erythema at the injection site, pain at the injection site, reactions at the injection site, including hemorrhage, hematoma, induration, swelling and itching);
- malignant neoplasms (non-melanoma skin cancer, malignant neoplasms of the prostate, intestines, mammary glands and melanoma in situ);
- hypersensitivity reactions (rash, urticaria).
Immunogenicity: Approximately 6% of patients receiving ustekinumab developed antibodies to the drug, which usually had a low titer. There was no clear correlation between the formation of antibodies and the presence of reactions with the injection. Most patients who had antibodies to ustekinumab also had antibodies that neutralized them. In the presence of antibodies to ustekinumab, patients often had lower efficacy of the drug, although the presence of antibodies does not exclude the achievement of a clinical effect.
In post-registration use of ustekinumab, adverse events from the immune system were identified: hypersensitivity reactions (including rash and urticaria), serious hypersensitivity reactions (including anaphylaxis and angioedema); from the skin and subcutaneous tissues: plaque psoriasis.
Pregnancy and lactation
It is not recommended to use the drug during pregnancy; effective methods of contraception should be used during and 15 weeks after treatment with the drug.
A decision should be made to stop breastfeeding while taking the drug or to discontinue ustekinumab therapy.
Contraindications
- Clinically significant hypersensitivity to ustekinumab or any excipient of the drug;
- Children's age (up to 12 years);
- Pregnancy and lactation;
- Serious infectious diseases in the acute phase, including tuberculosis;
- Malignant neoplasms.
Carefully
- Chronic or recurrent parasitic and infectious diseases of a viral, fungal or bacterial nature.
- History of malignant neoplasms.
- Elderly age.
Overdose
During clinical studies, patients were given single intravenous doses of up to 6 mg/kg without the development of dose-limiting toxicity. In case of overdose, it is recommended to monitor the patient's condition for signs and symptoms of side effects and, if they develop, appropriate symptomatic therapy should be started immediately.
special instructions
- Ustekinumab is a selective immunosuppressant and may increase the risk of developing infections and reactivation of latent infections. In clinical studies, serious bacterial, fungal and viral infections were observed in patients using ustekinumab. Ustekinumab should not be used in patients with clinically significant, active infections. Caution should be exercised when using the drug in patients with chronic infections or a history of recurrent infections.
- Before starting to use the drug, the patient should be tested for the presence of tuberculosis. Ustekinumab should not be used in patients with active tuberculosis. If you have latent or active tuberculosis (including a history of tuberculosis), treatment should be started before using ustekinumab. Treatment of tuberculosis should also be started in patients in whom the sufficient effect of previous treatment is unconfirmed. During and after treatment with ustekinumab, patients should be closely monitored for signs and symptoms of active tuberculosis.
- Patients should be warned to seek medical attention if signs and symptoms suggestive of infection appear. If a serious infection develops, the use of ustekinumab should be discontinued and the patient should be under the supervision of medical personnel. Ustekinumab should not be used until treatment for the infection has been completed.
- Immunosuppressants may increase the risk of developing malignancies. In some patients receiving ustekinumab in clinical trials, the occurrence of malignant neoplasms (cutaneous and non-cutaneous forms) was observed. The use of ustekinumab has not been studied in patients with a history of malignancy. Caution should be exercised when prescribing the drug to patients with a history of malignancy, as well as when considering continued treatment with ustekinumab in patients diagnosed with malignancy. All patients over the age of 60 years, as well as those who have previously received long-term therapy with immunosuppressants or UV radiation, should be screened for the presence of non-melanoma skin cancer.
- Serious hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during post-marketing use of ustekinumab. If anaphylactic or other serious hypersensitivity reactions occur, ustekinumab should be discontinued immediately and appropriate treatment should be instituted.
- The patient should not be vaccinated with live vaccines during the period of treatment with ustekinumab, as well as in the period 15 weeks before vaccination (after taking the last dose of the drug) and 2 weeks after vaccination. Caution should be exercised when using live vaccines to immunize family members of a patient receiving ustekinumab, since there is a risk of viral or bacterial release and transmission of infection from these individuals to patients. Long-term treatment with ustekinumab does not suppress the humoral immune response to vaccines containing pneumococcal polysaccharide and tetanus vaccine. Vaccines containing inactivated microorganisms can be used with ustekinumab, but the induced immune response may not be sufficient to prevent disease.
- Concomitant immunosuppressive therapy: The safety and effectiveness of ustekinumab in combination with immunosuppressive drugs and phototherapy have not been studied in patients with psoriasis. In studies in patients with psoriatic arthritis, coadministration with methotrexate did not affect the safety and efficacy of ustekinumab. Caution should be exercised when considering the possibility of simultaneous
- the use of other immunosuppressants and ustekinumab, as well as when switching from therapy with another antipsoriasis biological drug to ustekinumab therapy.
- Use in elderly patients (over 65 years): Of the 4031 patients treated with ustekinumab, 248 were patients over 65 years of age (183 patients with psoriasis and 65 with psoriatic arthritis). In clinical studies, there was no effect of age on clearance or volume of distribution of the drug. Although studies of the drug showed no differences in the safety and effectiveness of the drug in elderly patients over 65 years of age compared with younger patients, the number of elderly patients is insufficient to make a definitive conclusion about the effect of age (or lack thereof) on clinical effectiveness.
Use in children: The safety and effectiveness of ustekinumab in children has not been studied. The drug has not been studied in patients with renal or hepatic impairment.
Special instructions.
Currently, there is data on the effectiveness and safety of continuous therapy with ustekinumab for 5 years in patients with moderate to severe psoriasis. Multicenter, double-blind, randomized, placebo-controlled studies have shown that the effectiveness of ustekinumab remains high with continuous use of the drug for 5 years, and the most common adverse events are upper respiratory tract infections.
Diagnosis of genital psoriasis or psoriasis on the genitals
Psoriasis on the labia in women and on the head of the penis in men is not a separate disease. This is just one of the areas where the rash may be located. The problem can be localized in other parts of the body, most often the knees, elbows, abdomen, chest, back, buttocks, scalp, and in this case diagnosis is not difficult. Only genital rashes are rare, but cause more concern due to complex diagnostics, and often such patients are prescribed many tests to exclude infectious and allergic diseases.
Psoriasis of any localization, including genital psoriasis, is characterized by the psoriatic triad:
- The “stearin stain” phenomenon.
- The phenomenon of “terminal film”.
- The phenomenon of "bloody dew".
Fig.1
If the psoriatic triad is doubtful, the doctor may suggest a histological examination.
Pathophysiology of psoriasis
According to modern concepts, psoriasis is a complex multifactorial pathology that develops under the influence of genetic and immune-mediated factors.
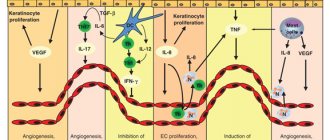
This hypothesis is confirmed by the success of immunodrugs in treating the disease. The pathogenesis of psoriasis is not fully understood. It is known that after exposure to triggers (and in some patients without them), a large number of leukocytes are sent to the dermis and epidermis. Primarily, the epidermis is infiltrated by many activated T cells, which are presumably capable of inducing keratinocyte proliferation. This theory is supported by data from histological studies and immunohistochemical staining of psoriatic plaques. American scientists have calculated that in a patient with damage to 20% of the body surface, 8 billion T cells circulate in the blood, while in the dermis and epidermis of psoriatic plaques their number reaches 20 billion [4].
Ultimately, an unregulated inflammatory process develops, which is accompanied by the release of a large number of various cytokines. Among them are TNF-alpha, interferon-gamma, interleukin-12 and others. Many of the clinical manifestations of psoriasis are associated with high levels of cytokines. Interestingly, elevated levels of TNF-alpha correlate with flare-ups of psoriasis, which has formed the basis for pharmacotherapy.
Further maintenance of T cell hyperactivity and the release of proinflammatory mediators play a major role in the pathogenesis of psoriasis [5].
In the affected areas of the skin, due to the expansion of superficial blood vessels, epidermal hyperplasia develops, which leads to a shortening of the cell life cycle (from 23 days to 3-5 days) and pathological cell maturation.
Epidermal cells, which normally lose their nuclei, retain them due to changes in the cell cycle—parakeratosis develops. In addition, affected cells do not release adequate amounts of lipids, which are necessary for the formation of a “cement” adhesion between keratinocytes. Against the background of lipid deficiency, a loose stratum corneum is formed, which leads to peeling with peeling of silvery scales.
Characteristic signs of psoriasis on the genitals
A patient with psoriasis on the genitals complains of suspicious rashes.
Regardless of the location, most psoriatic rashes appear as a cluster of rich pink or red papules, the top of which is covered with white scales.
Genital psoriasis in women is predominantly associated with hormonal changes in the body that occur during puberty, pregnancy and the onset of menopause. A rash with psoriasis in women appears on the skin of the labia majora, in the pubic area, and inguinal folds. Sometimes psoriasis affects the mucous membranes of the labia minora. The disease can be recognized by the characteristic features of the rash: papules usually have a clear oval or circle shape, their boundaries are clearly visible, covered with scales on top, itching is almost always absent. Even though there are lesions in other parts of the body, women usually do not perceive this disease as psoriasis. Representatives of the fairer sex more often identify rashes as a manifestation of gynecological infections.
In men, genital psoriasis primarily affects the penis and head of the penis; the rash can also be located in the folds and pubic area. Usually the rashes are pink or red papules, slightly protruding above the surface of the skin, having smooth borders and covered with scales on top. Peeling and itching are usually absent. In advanced situations, the rash spreads to the inner surface of the skin covering the penis. Such phenomena cause particular discomfort among representatives of the stronger sex.
When a man or woman is diagnosed with genital psoriasis, treatment should not be delayed. Since this area is subject to mechanical friction, the skin in the groin area can become even more traumatized, its surface will begin to crack, which leads to the spread of rashes and damage to an increasingly larger area.
IMPORTANT! Psoriasis on the genitals threatens the normal sexual life of men and women. This, in turn, can trigger neuropsychiatric disorders, which will require more serious correction, so treatment should be started in a timely manner.
Differential diagnosis of non-infectious changes in the penis
The content of the article
| Changes | Characteristic symptoms | Typical clinical picture | Differential diagnosis |
| Papulosquamous | |||
| Psoriasis | itching | red or salmon-colored, papular desquamating, ring-shaped plaques often associated with white or silvery scales; similar changes outside the genital area | cancer in place |
| Penile papules | asymptomatic | small, dome-shaped, flesh-colored lumps arranged in a ring shape | lichen planus, angiokeratoma |
| Inflammatory changes | |||
| Lichen sclerosus (balanitis xerotica obliterans ) | phimosis, painful erections, difficulty urinating, itching, pain, bleeding | hypopigmented, thinned foreskin, reminiscent of phimosis, cellophane-like surface; the changes are arranged in the shape of a ring or geometric shapes; lesions limited to foreskin and glans | carcinoma in situ , leukoplakia, scleroderma |
| Ringworm | asymptomatic | pinhead-sized papules with hypopigmentation, often in the extragenital area | HSV infection, pearly penile papules |
| Lichen planus | itching, soreness | at the top there are flattened, polygonal, purple lumps; often in extragenital areas | secondary syphilis |
| Vascular changes | |||
| Angiokeratoma | asymptomatic | red or blue lumps, often in the shape of a ring or geometric shapes; they can appear only on the head or also on the scrotum, groin, thighs or abdominal wall | penile papules |
| tumor changes | |||
| Carcinoma in situ (Keirat's erythroplasia, Bowen's disease) | itching, pain | varied clinical picture; plaques or ulcers limited to the glans, foreskin, or other areas of the penis; biopsy is necessary to make a diagnosis | psoriasis, lichen sclerosus, Zoon's balanitis, invasive penile cancer, HSV infection, syphilis, group B streptococcal balanitis, candidiasis, reactive arthritis |
| Invasive squamous cell carcinoma | late onset of symptoms, usually painless | exophytic or endophytic changes; the clinical picture is varied; the changes are local or metastatic; a biopsy is necessary to make a diagnosis | giant condylomas (HPV infection) |
Itching
Treatment of genital psoriasis in men and women
Treatment regimens for genital psoriasis differ from generally accepted methods for eliminating rashes on other parts of the body. The difference is that the genitals have special sensitive areas with mucous membranes; the effect in this area must be especially delicate and careful.
As a rule, for genital psoriasis, complex treatment is prescribed. The optimal therapy should be selected by the doctor, based on medical history, the general condition of the patient, and his individual characteristics. The principles of treatment depend on the degree of the disease and the severity of symptoms.
Typically, a regimen of external therapy for genital psoriasis or psoriasis on the genital organs involves prescribing the following groups of drugs:
- hormonal drugs - such drugs help relieve inflammation and reduce rashes, but they are not recommended for long-term use;
- non-hormonal agents based on activated zinc pyrithione;
- softening and caring components - they help moisturize the skin in the delicate area, eliminate signs of peeling, and restore the natural lipid barrier.
External hormonal preparations should be used with caution, because the surface of the genitals is highly absorbable.
Anatomical differences in absorption (% of the total absorbed dose from the entire
body surface area) are:
- Plantar surface of the foot - 0.14%;
- Palmar surface - 0.83%;
- Forearm - 1.0%;
- Scalp - 3.5%;
- Forehead - 6.0%;
- Lower jaw area - 13%;
- The surface of the genitals is 42%.
The use of external hormones inhibits local immunity, which enhances the growth of fungal flora. After a course of therapy, it is important to gradually reduce the use of hormonal drugs to avoid withdrawal syndrome.
Preparations with the irritating effect of tar and petroleum derivatives are not recommended for use in psoriasis on sensitive areas and genitals, as they have side effects such as severe skin irritation (including burns), persistent discoloration of the skin, increased sensitivity of the skin to the sun (phototoxicity) , frequent cases of allergies. Also, these groups of drugs are characterized by low compliance due to an unpleasant odor.
Many specialists prefer to treat genital psoriasis with non-hormonal Skin-cap cream. Skin-cap cream contains activated zinc pyrithione, which has a pronounced anti-inflammatory property and antipruritic effect. Additionally, the formula of the drugs includes moisturizing components. Skin-cap cream can also be used to prevent progression, for example, to avoid the appearance of psoriasis rashes on the pubis after depilation or shaving.
General principles of treatment
Complex treatment of psoriasis in the upper and lower extremities includes the use of the following methods:
- Drug therapy, including the use of drugs for oral and external use. For therapeutic purposes, tableted cytostatics are prescribed, and for external application to areas of psoriatic rashes, ointments and creams with glucocorticosteroids, phytoextracts and local anesthetics are used. Also, sprays that have anti-inflammatory, cooling and decongestant effects are widely used.
- Sanatorium-resort treatment (therapeutic baths, mud applications).
- Carrying out photochemotherapy.
- Physiotherapeutic treatment.
- Compliance with dietary recommendations and taking multivitamin complexes.
To treat psoriasis of the nail plates, the following drugs are used for external application:
- Advantan cream.
- Dayvonex ointment.
- Beloderm ointment.
- Anthralin cream or ointment.
Each product is applied to damaged areas 2 times a day and left until completely absorbed.
Medicines containing vitamin D3 help slow the progression of this disease. To prevent relapse of psoriasis, Xamiol gel, Rocaltrol capsules and Daivobet ointment can be used. Dermatotropic agents such as zinc and salicylic ointment help slow down the rate of skin cell division. Zorak gel or Tazorac cream will help reduce the intensity of inflammation.
Additional Relapse Prevention Recommendations
Psoriasis on the genitals cannot be completely cured, but you can take control of this disease and reduce the likelihood of exacerbations. To do this, it is necessary not only to undergo timely medical examinations and follow doctors’ recommendations, but also to reconsider your lifestyle. Here are some recommendations to reduce the likelihood of relapses of genital psoriasis:
- review the diet, exclude foods that cause aggravation;
- provide high-quality and regular skin care in the intimate area;
- exclude traumatic procedures (epilation, shaving, aggressive peelings in the bikini area);
- give preference to linen made from natural soft fabrics (cotton is ideal);
- try to avoid stress, balance activity and rest.
With an integrated approach to prevention and treatment, genital psoriasis can be kept under control, thereby increasing the quality of life of patients.
Fig.1. Psoriatic triad
Rice. 2. Psoriasis on the penis
Lichen sclerosus
Lichen sclerosus of the penis, also known as balanitis obliterans, can occur at any age. The average age of patients at diagnosis is 42 years, and the incidence is 1 in 300 men. In 4–6% of patients, lichen sclerosus is associated with the development of squamous cell carcinoma. Genital lichen sclerosus is considered a precancerous condition.
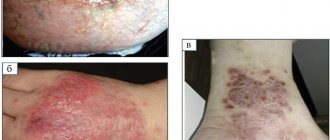
Lichen sclerosus appears as a hypopigmented lesion with a surface resembling wrinkled paper or cellophane. It mainly affects the glans penis and foreskin. There may be visible blisters, erosions and skin atrophy. Patients usually have phimosis, painful erections, difficulty urinating, itching, pain and bleeding.
Photo 1. Lichen sclerosus
Photo 2. Lichen sclerosus
Because skin lesions can obstruct the urethra, the first symptom in patients may be urinary retention. Although lichen sclerosus can affect almost any part of the body, some people may have no symptoms. It must be differentiated from carcinoma in situ, leukoplakia, and systemic sclerosis. If squamous cell carcinoma cannot be ruled out, a biopsy is indicated.
- In the treatment of lichen sclerosis, topical fluorinated glucocorticoids with moderate to very high potency are usually used to reduce symptoms and prevent malignant transformation.
- With very high efficacy, GCS pulse therapy is effective and safer than long-term daily use of weaker GCS.
- Tacrolimus or pimecrolimus may also be effective, but their safety for long-term use has not been established.
Surgery is indicated for persistent lesions or if there is clinical suspicion that changes in the penis may be squamous cell carcinoma. In uncircumcised patients with lichen sclerosus limited to the glans and foreskin, removal of the foreskin (circumcision) may be indicated. For severe lesions, reconstructive surgery may be required, although if the risks of surgery outweigh the potential benefits, conservative treatment may be appropriate.
Systemic medications such as retinoids and methotrexate are indicated for the treatment of severe lichen sclerosus or when topical treatments are ineffective. Long-term follow-up with periodic physical examination is necessary to monitor the patient for malignant transformation.
Causes of psoriasis
Both the skin and the entire human body as a whole participate in the process of plaque formation. The causes may be viral, infectious, hereditary, psychosomatic or mixed.
- constant nervous stress, breakdowns, depression;
- genetic predisposition;
- hormonal imbalance;
- allergic reactions;
- diseases of the gastrointestinal tract;
- liver diseases;
- metabolic disease;
- viral, bacterial, fungal infections;
- frequent mechanical injury and irritation.
Psoriasis can appear spontaneously and also disappear spontaneously. Since the disease is chronic, there are periods of exacerbation and remission.
Human skin can act as a mirror that reflects the physiological and emotional state of a person. The skin is sensitive to changes in the body. This is called psychosomatic causes of psoriasis. Therefore, when treating a disease, it is worth paying attention to the psychological state of the patient.