Author
: Grachev Ilya Illarionovich
Editor
: Efremov Mikhail Mikhailovich
Date of publication: 10/22/2018 Date of update: 06/08/2020
- Drug therapy
Palmoplantar psoriasis is one of the most common forms of this chronic skin disease. The most functionally loaded parts of our body are affected: the palms and feet, on which the main burden falls when working and walking. From this article you can learn how to help a patient identify the disease in a timely manner and where to go for qualified treatment. And also everything about the treatment of this disease at the Moscow Paramita clinic.
Causes of palmoplantar psoriasis
Exactly why and how the disease develops is still unknown. But the basis of any form of psoriasis is a hereditary predisposition: metabolic characteristics leading to changes in immunity and the development of autoimmune (with allergies to the body’s own tissues) processes.
Psoriasis of any localization can spread throughout the body, so do not delay treatment.
See how easily the disease can be cured in 10-12 sessions.
Anyone who has close relatives suffering from psoriasis is at risk. But not all people at risk develop palmoplantar psoriasis. Predisposing or risk factors play a major role here. They can be divided into general and local. General risk factors contribute to the development of any form of psoriatic process. This:
- hormonal disorders;
- the presence of any chronic diseases and foci of infection that affect metabolism;
- the presence of allergic diseases, often activating autoimmune processes;
- the presence of chemical dependence (alcoholism, drug addiction, smoking) – dependence has a negative effect on the circulatory system and metabolism;
- prolonged stress and borderline neuropsychiatric diseases (neuroses) - the central nervous system (CNS) is the command post of our body and any changes in it cannot but affect the state of the immune system;
- heavy physical activity;
- improper malnutrition, consumption of predominantly high-calorie foods;
- long-term use of certain medications: antibiotics, lithium preparations, certain high blood pressure medications (ACE inhibitors, beta blockers), hormonal drugs, etc.
Local risk factors contributing to the development of psoriasis on the feet and palms:
- work associated with constant contact with water and detergents: dishwashers, bathhouse attendants, pool workers, etc.;
- use of household and industrial chemicals;
- previous skin infections - fungus of the hands, feet, interdigital spaces, streptococcal impetigo, herpetic infection, etc.;
- allergic skin diseases – autoimmune dermatitis, eczema;
- injuries, abrasions, abrasions on the palms and soles;
- excessive sweating of the feet, wearing tight shoes (plantar psoriasis).
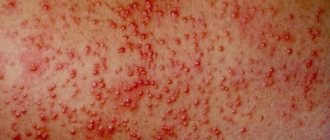
Red spots on the skin after stress
Diagnosis of skin diseases
The cause of the onset of palmoplantar psoriasis is usually the effect on the body of not one, but several factors at once. Therefore, it is so important for people at risk to know these factors and eliminate their impact on the body.
Symptoms of palmoplantar psoriasis
Symptoms of palmoplantar psoriasis can vary significantly from patient to patient.
Forms of the disease
Plaque-fan-shaped (vulgar)
. It is manifested by the formation of characteristic psoriatic papules on the skin of the palms and soles - clearly demarcated, pink, raised above the surface of the skin and covered with yellowish scales. The elements of the rash are arranged fan-shaped in the form of rays and gradually merge into large round plaques. The skin of the palms and feet is dry, cracks often appear on them, which facilitates infection.
Circular
. With this form, dry skin and itching first appear, and then characteristic circular rashes on the palms and soles, which often spread to the back of the hands and feet. This form is characterized by the appearance of large foci of peeling.
Horny or callous
. On the surface of the palms and soles, areas of increased keratinization appear with the formation of large, dense, dry calluses that merge with each other and cover the entire surface of the affected areas, moving to the sides.
Barber's pustular psoriasis
. Large, clearly defined plaques appear on the palms and soles, and on them there are sterile (without infection) pustular rashes (pustules) with a diameter of 2–5 mm. Sometimes they merge with each other, forming widespread purulent lakes. As the pustules dry out, they turn into brown crusts. The process is accompanied by severe itching.
Types of analyzes
The main tests performed for psoriasis include:
- Skin biopsy, a piece of skin is examined to differentiate bacterial, fungal infections, and cancer.
- Complete blood count to detect leukocytosis and anemia.
- Erythrocyte sedimentation rate to determine the type of psoriasis. With pustulosis and erythroderma, the indicator remains normal.
- Uric acid test to rule out gout.
- An HIV antibody test is performed because sudden onset of psoriasis can be caused by HIV infection.
- Skin pH testing helps evaluate the effectiveness of the therapy.
If psoriasis affects the joints (arthropathic form), a rheumatoid factor test is performed. It allows you to differentiate rheumatoid arthritis, in which the protein level is increased. Contrast arthrography and pneumoarthrography also help to assess the degree of joint damage.
Stages of the disease
Regardless of the form of palmoplantar psoriasis, its course is wavy. Exacerbations are replaced by remissions. The following types of disease course are distinguished:
- rarely recurrent - relapses occur every few years:
- moderately recurrent - relapses every 1-2 years;
- often recurrent - relapses every few months.
In most patients, exacerbations are seasonal - rashes appear only in the warm or only in the cold season. In this case, they talk about the summer or winter type of the disease. If exacerbations occur at any time of the year, the patient has a mixed type.
During an exacerbation (relapse), changes in the skin go through three stages:
- initial or progressive - elements of the rash spread and merge with each other, forming large conglomerates;
- stationary - the spread of the rash stops and it remains in the same state for a long time;
- regressive or restorative - healing occurs, the rash gradually turns pale and disappears, leaving first flaky spots or crusts on the palms and soles, and then pigmented (brownish) or depigmented (white) spots.
Complications of palmoplantar psoriasis
The main danger is the addition of an infection, which can significantly complicate the course of psoriasis. The pathological process can spread to other parts of the body, as well as to the joints (psoriatic arthritis) and internal organs.
A severe lesion is psoriatic erythroderma. The skin of the entire body turns red and becomes covered with flaky plates. The condition is serious, there is itching, a feeling of tightness, and fever. Such patients require urgent hospitalization.
Epidemiological situation
Based on the causes of its occurrence, psoriasis is a non-infectious disease with a pronounced genetic predisposition. The people most at risk of getting sick are those whose relatives also suffer from this disease (in this case, we mean only the closest relatives). European researchers have established for certain that if one of the parents is sick, he has a 14-25% chance of passing it on to his child. If both parents are sick, this probability is already 41-60%.
Based on the type of disease development, psoriasis is divided into two groups:
- early;
- late.
This is evidence that there are two main types of psoriasis (like diabetes mellitus, for example). The first occurs in humans at an early age (on average 16-22 years), is strictly hereditary in nature and is directly related to the HLA phenotype (HLA-Cw6). The course of the disease is often severe and the disease only progresses over time.
The causes of type 2 psoriasis are rather random in nature, so this disease is sporadic. Occurs most often in older people (about 60 years old). In general, it proceeds quite easily, however, in some cases, it can be aggravated by damage to the joints and nails.
How is palmoplantar psoriasis treated?
Treatment should be comprehensive and include:
- healthy active lifestyle;
- proper nutrition;
- drug treatments;
- non-drug treatment methods;
- traditional methods of treatment.
Drug therapy
General and local drug therapy is prescribed. For mild cases of the disease, general treatment includes:
- desensitizing agents that reduce the manifestations of allergies and itching:
- calcium gluconate;
- antihistamines – Claritin, Erius, Tavegil, Suprastin, etc.
Mild to moderate palmoplantar psoriasis can be treated with external remedies:
- ointments, creams and solutions based on corticosteroid hormones; relieves allergic and inflammatory processes, itching;
- combined agents (hormones with antibiotics); prescribed when a bacterial infection occurs;
- ointments with a synthetic analogue of vitamin D (Davonex) - inhibit the reproduction (proliferation) of surface epithelial cells;
- combined ointments with hormones in combination with vitamin D analogues (Daivobet);
- preparations with retinoids (analogs of vitamin A) - suppress peeling well;
- keratolytics (salicylic acid), tar-based emollients and combination products containing hormones and salicylic acid (Diprosalic); these remedies are well suited for the calloused form of palmoplantar psoriasis;
- pastes with active zinc – have an anti-inflammatory effect.
Non-drug treatments
Good to know
- Treatment of psoriasis on the head
- Treatment of psoriasis on the elbows
- Treatment of psoriasis on the legs
- Treatment of psoriasis on nails
- Treatment of psoriasis on the hands
- Treatment of psoriasis on the face
These methods of treating palmoplantar psoriasis include modern European and ancient Eastern techniques, widely used by dermatologists to suppress the psoriatic process. But when choosing a clinic, you should pay attention to whether the doctors have training in certain techniques, and where they received it. In Russia you can get good training in European methods of non-drug treatment. And it is best to study traditional eastern methods of treatment in China or Tibet. The best clinics in Moscow offer the following methods:
- Reflexotherapy is the effect on active points on the body using various methods: acupuncture (Zhen method), cauterization with wormwood cigarettes (Ju method), massage, vacuum (cans), compression with metal balls and plates, etc. By influencing certain points, you can have a reflex effect on any organ. The method has been proven for centuries, but is very difficult to implement. It is performed well mainly by specialists trained in China.
- Herbal medicine is the use of medicinal plants to treat diseases. The method also requires special preparation, but it allows you to reduce the drug load on the body by partially replacing drugs with herbs.
- PRP therapy is the injection of the patient’s own blood, taken from a vein and then enriched with platelets, into the affected areas of the skin of the palms and soles. The technique makes it possible to transfer the course of the disease to the third regressive stage, since platelets perform the function of restoration. High effect: just a few procedures can stop the spread of the psoriatic process.
- Autohemotherapy is treatment with the patient’s own blood. The method has long been used in the treatment of psoriatic processes and has proven itself well. Intramuscular injection of venous blood from a patient shakes up the body and forces it to fight the disease.
How palmoplantar psoriasis is treated at the Paramita Clinic
The Paramita Clinic specializes in Eastern methods of treating palmoplantar psoriasis. The clinic's dermatologists were trained in reflexology and herbal medicine in China and Tibet. Therefore, they can relieve the main symptoms of the disease without the use of drugs.
To avoid relapses, it is necessary to eliminate the cause of the disease.
Read more about our unique method of treating psoriasis
But this does not mean that drug therapy and modern European treatment methods are not used in the clinic. All doctors are trained in these methods. A wide selection of methods for treating palmoplantar psoriasis in Moscow, the use of folk remedies and extensive practical experience allow specialists to select individual therapy for each patient and quickly and effectively eliminate the main symptoms of the disease. To prevent exacerbations, preventive courses of treatment are carried out.
This approach allows the clinic’s patients to forget about exacerbations for a long time and live a normal life without experiencing pain.
Palmoplantar psoriasis is a disease that requires timely treatment and prevention of exacerbations. If this is not done, a decrease in quality of life is guaranteed. But modern methods allow such patients to forget about exacerbations and live a normal life. Psoriasis of the hands and feet can be treated well if treated by a specialist.
Sign up for a free initial appointment
Is psoriasis dangerous?
Psoriasis is a serious but not fatal disease. It reduces the quality of life because it is unsightly in appearance. Plaques on the body prevent patients from working in a team or resting. They often lead to limited movement and difficulty performing simple physical tasks. Untimely treatment of psoriasis can lead to damage to the organs of vision and joints.
It is impossible to cure psoriasis completely. This is a chronic dermatological disease that must always be kept in a “dormant” state.
Proper nutrition for palmoplantar psoriasis?
Nutrition is of great importance in palmoplantar psoriasis. There is no special diet, but it is recommended to adhere to certain principles:
- eat regularly 4 – 5 times a day in small portions;
- exclude from the diet: high-calorie foods - fatty meats and dairy products, confectionery, sweets;
- plants from the nightshade family: tomatoes, eggplants, hot and bell peppers;
- all fried, spicy, salted, smoked, canned foods;
- more vegetables and fruits;
Advantages of taking tests in the laboratory of JSC "SZDCM"
- Easy registration and no queues.
- Anonymity and confidentiality of data.
- Accurate diagnostics thanks to the latest equipment.
- Quick availability of results.
- Tactful, qualified staff.
Laboratory terminals operate in St. Petersburg and the Leningrad region, Pskov, Veliky Novgorod and Kaliningrad. You can get tested at any of them, regardless of your place of registration and residence. The laboratories have a single database, which means you can get the result in any department, in a way convenient for you.
Is it possible to use folk remedies to treat palmoplantar psoriasis?
Psoriasis is a complex disease that has not been fully studied, so medicine does not recommend treating it independently, including with folk remedies: instead of improvement, the pathological process can progress. But dermatologists often and successfully use folk remedies, replacing drug therapy with them. It is important that treatment of plantar psoriasis with folk remedies is prescribed by a specialist, taking into account the form and stage of the disease, as well as the general condition of the patient. Here are some folk recipes:
- baths with herbal infusion for hands or feet; take 5 tablespoons of celandine herb and chamomile flowers, add a liter of hot water, bring to a boil, boil for 5 minutes, strain, dilute with a liter of cool water and use for local baths;
- olive oil for lubricating feet; In the morning, lubricate the soles of your feet with warm olive oil; this promotes hydration and prevents cracking.
Biological drugs created using genetic engineering methods are monoclonal antibodies used for therapeutic purposes. In domestic medical practice, the following biological drugs are approved for medical use for the treatment of psoriasis: infliximab, adalimumab, ustekinumab, etanercept.
1. Infliximab
Infliximab is a selective TNF-a antagonist, which is a chimeric monoclonal IgG antibody that consists of 75% human and 25% mouse protein. Infliximab is indicated for the treatment of adult patients with psoriasis with severe and moderate forms of the disease in the absence of clinical effect from the use of other systemic therapies (including cyclosporine, acitretin, methotrexate and PUVA therapy) or in cases of intolerance or contraindications to their use, as well as for the treatment of active progressive psoriatic arthritis.
Doses and regimens
The drug is administered intravenously by drip for at least 2 hours at a rate of no more than 2 ml/min. under the supervision of medical personnel. For the treatment of psoriasis and psoriatic arthritis, the initial dose of infliximab is 5 mg per kg of patient weight. After the first administration, the drug is administered in the same dose after 2, then 6 weeks. and then every 8 weeks. If there is no effect within 14 weeks. (after four intravenous infusions) it is not recommended to continue treatment. During the intravenous infusion and for at least 1-2 hours after its completion, the patient must be under the supervision of a physician. During intravenous infusion of the drug, it is necessary to measure blood pressure, pulse, and respiratory rate and body temperature every 30 minutes.
Adverse reactions/safety
In clinical studies, adverse reactions were observed in approximately 60% of patients receiving infliximab and 40% of patients receiving placebo.
- Infusion reactions occur during infusions or within 1-2 hours after it. These include swelling of the pharynx/larynx, bronchospasm, chills, headache, hot flashes, nausea, shortness of breath. In clinical trials, the incidence of infusion reactions when using infliximab was about 20%, in the comparison group (placebo) - about 10%. Approximately 3% of patients were forced to discontinue treatment due to the development of infusion reactions, which were reversible in all patients (with or without drug therapy).
- Delayed hypersensitivity reactions in the form of arthralgia, myalgia, fever and rash develop in 1% of patients with psoriasis at the beginning of treatment.
- In clinical trials, when infliximab treatment was repeated 2-4 years after the previous course of therapy, patients experienced adverse reactions (myalgia, arthralgia accompanied by fever and/or rash, itching, swelling of the face, lips or hands, dysphagia, urticaria, pain sore throat and/or headache), which developed 3-12 days after re-infusion.
- Infectious complications are the most common serious side effects. They were associated with approximately 50% of all recorded deaths. There have been cases of tuberculosis, including miliary tuberculosis with extrapulmonary localization, in some cases with a fatal outcome.
- Malignant neoplasms and lymphoproliferative diseases. There have been cases of emergence or recurrence of malignant neoplasms. The incidence of lymphoma in patients treated with infliximab was higher than the expected incidence of this disease in the general population. The incidence of other forms of malignant neoplasms in patients treated with infliximab did not exceed the reported incidence in the general population.
- Cardiovascular failure. Cases of progression of cardiovascular failure during the use of infliximab have been described. There are rare reports of newly diagnosed cardiovascular failure, including in patients who had no previous diseases of the cardiovascular system.
- Changes in the liver and biliary tract. There are very rare reports of the appearance of jaundice and non-infectious hepatitis, in some cases having signs of autoimmune hepatitis, the development of liver failure, leading to the need for a liver transplant or fatal outcome. A cause-and-effect relationship between the occurrence of these adverse reactions and treatment with infliximab has not been established. There have been cases of exacerbation of hepatitis B in patients who were chronic virus carriers (who had a positive reaction to HBsAg).
- When treated with infliximab, a mild to moderate increase in aminotransferase activity may be observed without the development of significant liver damage. In most cases, the increase in aminotransferase levels is transient and asymptomatic. A decrease or return to the initial level of these indicators occurs regardless of whether treatment with infliximab is continued or stopped or concomitant therapy is changed. An increase in alanine aminotransferase activity to a level equal to or greater than 5 times the upper limit of normal was observed in 1% of patients.
- When using infliximab, isolated cases of the development of demyelinating diseases of the central nervous system, optic neuritis, and epileptic seizures have been described.
- In some patients, antinuclear antibodies appeared in the blood serum during treatment with infliximab. Cases of reversible lupus-like syndrome have been described.
Pregnancy/lactation
Infliximab is not recommended for use during pregnancy. During treatment with the drug and for 6 months. after its completion, reliable methods of contraception should be used. Breastfeeding should be discontinued when infliximab is prescribed. Breastfeeding is allowed no earlier than after 6 months. after the end of therapy.
Prevention and treatment of adverse reactions
For early detection of an acute infusion reaction, the patient should be carefully observed during and for at least 1-2 hours after the drug infusion. If an acute infusion reaction occurs, the drug should be discontinued. When conducting infliximab infusions, it is necessary to have appropriate equipment and medications (adrenaline, glucocorticosteroids for parenteral administration, antihistamines, equipment for artificial ventilation). To prevent mild and transient infusion reactions, the patient may be prescribed antihistamines before starting the infusion.
Contraindications/restrictions
- Hypersensitivity reactions to infliximab, other murine proteins, as well as to any of the inactive components of the drug.
- Severe infectious process, for example, sepsis, abscess, tuberculosis or other opportunistic infection.
- Heart failure - severe or moderate.
- Pregnancy and breastfeeding.
- Age less than 18 years.
Interaction
The interaction of infliximab with other drugs has not been studied. Concomitant use with methotrexate in patients reduces the formation of antibodies to infliximab and increases its concentration in plasma.
Overdose
A single administration of infliximab in doses up to 20 mg/kg did not cause direct toxic effects. In case of overdose, observation and immediate relief of symptoms are necessary.
special instructions
- Infliximab, when administered, can cause the development of acute allergic reactions (immediate type) and delayed allergic reactions. Acute infusion reactions may develop immediately or within several hours after administration. A patient receiving infliximab should be monitored during and for at least 1-2 hours after the drug infusion.
- Some patients may develop antibodies to infliximab, which is associated with a more frequent development of infusion reactions. In patients suffering from Crohn's disease, there has been a correlation between the formation of antibodies and a decrease in the duration of the effect of treatment. Patients who stop taking immunosuppressive drugs before or during treatment with infliximab are more at risk of developing these antibodies. If severe reactions develop, symptomatic therapy should be carried out, and further use of the drug should be excluded.
- As the interval between infusions increases, the likelihood of the formation of antibodies to infliximab increases. When re-prescribing infliximab after a long break in treatment, it is necessary to be wary of the appearance of a delayed-type hypersensitivity reaction in the patient.
- When prescribing infliximab to patients with a history of indications of malignant neoplasms, or when deciding whether to continue treatment with infliximab in patients with newly diagnosed neoplasms, special caution should be exercised.
- Before starting treatment with infliximab, the patient should be carefully examined to identify both active and latent tuberculosis. The examination should include:
- careful collection of anamnesis (presence of tuberculosis in the past and/or contact with tuberculosis patients);
- X-ray examination of the chest in two projections;
- conducting a tuberculin test;
- consultation with a phthisiatrician.
- If a tuberculous process is suspected, the use of infliximab should be discontinued until diagnosis is made and, if necessary, appropriate therapy should be administered.
- The patient should be informed that he needs to consult a doctor if the following symptoms appear during treatment with infliximab or after its completion: cough, weight loss, low-grade body temperature.
- During treatment and after its completion, the patient should be closely monitored for signs of possible infection. Since elimination of infliximab occurs within 6 months, the patient should be constantly under medical supervision during this period. Treatment with infliximab should be discontinued if the patient develops a severe infection, including tuberculosis, sepsis or pneumonia.
- The use of live vaccines during treatment with infliximab is not recommended.
- In rare cases, an autoimmune process may develop in genetically predisposed patients. In the event of persistent rash, fever, joint pain, fatigue, or the presence of DNA antibodies in the blood, treatment with infliximab should be discontinued.
- The benefits and risks of infliximab should be carefully weighed in patients with pre-existing or new-onset CNS demyelinating disease.
- Patients with moderate circulatory insufficiency should be carefully monitored. If symptoms of circulatory failure increase, infliximab should be discontinued.
- Patients with signs of liver dysfunction should be evaluated for liver disease. If jaundice occurs or alanine aminotransferase activity increases to levels greater than 5 times the upper normal value, infliximab should be discontinued and a thorough evaluation should be performed.
- Hepatitis B virus carriers should be examined before treatment with infliximab and continuously monitored during treatment in order to timely detect a possible exacerbation of the disease.
- The effectiveness and safety of treatment with infliximab in children and adolescents under the age of 18 years, inclusive, suffering from psoriatic arthritis and psoriasis have not been studied. Until convincing data are obtained, the drug should not be used in these age groups.
- There have been no specific studies on the use of infliximab in elderly people, as well as in people with liver and kidney diseases. There is limited experience indicating the safety of treatment with infliximab in patients undergoing arthroplasty.
Monitoring laboratory parameters during treatment with infliximab
| Infusion weeks | ||||
| Before | 2nd week | 6th week | Every 8 weeks | |
| General blood test1 | X | X | X | X |
| General urine analysis | X | X | X | X |
| ALT,AST | X | X | X | X |
| Pregnancy test | X | |||
Note. 1 Hemoglobin, hematocrit index, erythrocytes, leukocytes, leukocyte formula, platelets.
2. Adalimumab
The selective immunosuppressive drug adalimumab is a completely identical human monoclonal antibody that blocks the activity of TNF-a, a pro-inflammatory cytokine that plays a key role in the pathogenesis of psoriasis. Doses and regimen. For chronic plaque psoriasis, the initial dose for adult patients is 80 mg. Maintenance dose - 40 mg once every 2 weeks, starting a week after the initial dose. The drug is administered subcutaneously into the thigh or abdomen.
Overdose.
The maximum tolerated dose of adalimumab in humans has not been established. Repeated use of adalimumab in doses up to 10 mg/kg was not accompanied by toxic effects requiring dose reduction. In case of overdose, it is necessary to monitor adverse reactions and immediately begin adequate symptomatic treatment.
Drug interactions
In patients with rheumatoid arthritis receiving methotrexate, there is no need for dose adjustment of adalimumab or methotrexate. However, methotrexate with single and repeated use reduces the clearance of adalimumab by 29% and 44%, respectively. The interaction of adalimumab with other drugs other than methotrexate has not been studied in pharmacokinetic studies. In clinical studies, there were no signs of interaction of adalimumab with other basic drugs (sulfasalazine, hydrochloroquine, leflunomide and parenteral gold preparations), corticosteroids, salicylates, NSAIDs and analgesics.
Pregnancy and lactation.
Adalimumab is contraindicated during pregnancy and breastfeeding. Adequate and strictly controlled studies of the drug in pregnant women have not been conducted. Women of reproductive age should avoid conceiving while being treated with the drug. Given the risk of serious side effects in the newborn, it is advisable to stop breastfeeding or discontinue the drug, taking into account its importance for the mother.
Side effects
- According to clinical studies, approximately 15% of patients can be expected to develop injection site reactions, which are one of the most common side effects with adalimumab in controlled clinical trials.
- Infections: very often - respiratory tract infections (including upper and lower respiratory tract infections, pneumonia, sinusitis, pharyngitis, nasopharyngitis and herpes viral pneumonia); often - generalized infections (including sepsis, candidiasis and influenza), gastrointestinal infections (including viral gastroenteritis), skin and soft tissue infections (including paronychia, cellulitis, impetigo, necrotizing fasciitis and herpes zoster), ear infections, oral infections (including herpes simplex, oral herpes and dental lesions), genital infections (including vulvovaginal mycotic infection), urinary tract infections (including pyelonephritis); uncommon - opportunistic infections and tuberculosis (including coccidioidomycosis, histoplasmosis and complex of infections caused by Mycobacterium avium), neurological infections (including viral meningitis), eye infections, bacterial infections, joint infections.
- Neoplasms: often - benign neoplasms, skin cancer, except melanoma (including basal cell carcinoma and squamous cell carcinoma); uncommon - lymphoma, parenchymal neoplasms, neoplasms of the mammary gland, lungs and thyroid gland, melanoma.
- From the blood and lymphatic system: very often - leukopenia (including neutropenia and agranulocytosis), anemia; often - thrombocytopenia, leukocytosis; uncommon - idiopathic thrombocytopenic purpura; rarely - pancytopenia.
- From the immune system: often - hypersensitivity reactions, seasonal allergies.
- Metabolism: very often - increased lipid levels; often - hypokalemia, increased uric acid levels, pathological changes in sodium content, hypocalcemia, hyperglycemia, hypophosphatemia, increased potassium levels in the blood; infrequently - dehydration.
- From the nervous system: very often - headache; often - paresthesia (including hypesthesia), migraine, sciatic neuralgia, mood changes (including depression), irritability, insomnia, dizziness; infrequently - tremor; rarely - multiple sclerosis.
- From the senses: often - conjunctivitis, visual impairment; infrequently - blepharitis, eyelid edema, diplopia, deafness, ringing in the ears.
- From the cardiovascular system: often - arterial hypertension, hot flashes, hematomas, tachycardia; uncommon - arrhythmia, congestive heart failure; rarely - cardiac arrest, arterial occlusion, thrombophlebitis, aortic aneurysm.
- From the respiratory system: often - cough, asthma, dyspnea; infrequently - chronic obstructive pulmonary disease, interstitial lung diseases.
- From the digestive system: very often - nausea, vomiting, abdominal pain, increased activity of liver enzymes; often - dyspepsia, gastroesophageal reflux, dry mouth (sicca syndrome), gastrointestinal bleeding; uncommon - pancreatitis, dysphagia, facial edema, cholecystitis, cholestasis, increased bilirubin levels, hepatic steatosis.
- Dermatological reactions: very often - rash (including exfoliative); often - itching, urticaria, hemorrhages (including purpura), dermatitis and eczema, brittle nails, hyperhidrosis; Uncommon: night sweats, scars.
- From the musculoskeletal system: very often - musculoskeletal pain; often - muscle spasms; uncommon - rhabdomyolysis; rarely - systemic lupus erythematosus.
- From the urinary system: often - hematuria, renal failure; infrequently - nocturia.
- From the reproductive system: infrequently - nocturia, erectile dysfunction.
- From laboratory indicators: often - violations of indicators
- blood clotting (including increased aPTT), positive tests for autoantibodies (including antibodies to the double helix of DNA), increased LDH levels.
- Local reactions: very often - reactions at the injection site (including erythema).
- Other: often - chest pain, swelling; infrequently - inflammation, deterioration of wound healing.
special instructions
.
Adalimumab has a number of important features. In particular, the drug has no risk of infusion reactions, since adalimumab is administered subcutaneously. The frequency of formation of antibodies to adalimumab is only 1.9-8.4%, which undoubtedly has a beneficial effect on both the safety profile of long-term use of the drug and has a positive effect on the level and duration of clinical response to treatment.
Long-term follow-up of psoriasis patients treated with open-label adalimumab over 3 years in the large REVEAL study is available. The effectiveness and safety of continuous therapy with adalimumab in patients with moderate to severe psoriasis for more than three years was assessed. The studies demonstrated that in patients with clinical responses to treatment PASI 75, PASI 90 and PASI 100, reflecting a decrease in the extent and severity of the skin process by 75%, 90%, 100%, respectively, the effectiveness of adalimumab was maintained for three years. Thus, after 160 weeks of continuous treatment with this biological agent, the percentage of patients with PASI 75 remained at 76%; PASI 90 - respectively 50%; PASI 100 - 31%. The data obtained suggest that the majority of patients with psoriasis who received adalimumab responded well to treatment carried out for more than 3 years. More than half of the observed individuals after 160 weeks of treatment with adalimumab had only minimal manifestations of psoriasis on the skin, and in one third of the patients the psoriatic process resolved completely. The most stable response to long-term treatment with adalimumab was found in patients with an improvement in PASI 100. The study made it possible to significantly clarify the data on a possible decrease in the effectiveness of the biological agent with long-term use. It was found that it is extremely important to assess response early in treatment with adalimumab. A good initial response rate with adalimumab (PASI 75 or more at weeks 16 and 33) is, in fact, a predictor of favorable long-term treatment outcomes over a follow-up period of 3 years or more. Finally, the study demonstrated the high safety of long-term use of adalimumab. It is important that among all study participants (n=1159), only 2 cases of tuberculosis were registered. Not a single case of lymphoma, lupus-like syndrome, or demyelinating disease was identified.
According to an analysis of the safety of adalimumab in psoriasis, where the assessment was based on data from 13 clinical studies, and the duration of treatment with the drug was up to 5 years, the frequency (expressed as the number of events per 100 PL) of serious AEs, severe infections, malignant neoplasms does not increase or even decreases as increasing the duration of treatment.
3. Ustekinumab
Ustekinumab is a fully human IgG1k monoclonal antibody that has high affinity and specificity for the p40 subunit of human interleukins (IL) IL-12 and IL-23. Ustekinumab is indicated for the treatment of patients over 18 years of age with moderate to severe plaque psoriasis, as well as patients with active psoriatic arthritis as monotherapy or in combination with methotrexate.
Directions for use and doses
Ustekinumab is intended for subcutaneous injection. The recommended dose is 45 mg. The second injection is given 4 weeks after the first application, then every 12 weeks. In patients weighing more than 100 kg, the drug is recommended to be used at a dose of 90 mg. For patients in whom the clinical effectiveness of the drug when used every 12 weeks is not sufficiently expressed, the dose of the drug should be increased to 90 mg every 12 weeks. If this dosing regimen is not effective, a dose of 90 mg should be administered every 8 weeks. Resumption of therapy according to the proposed regimen - a second injection 4 weeks after the first use, and then every 12 weeks - was as effective as the first therapy .
Side effects
The following side effects were observed in clinical studies of ustekinumab:
- infections (odontogenic infections, upper respiratory tract infections, nasopharyngitis, inflammation of subcutaneous fat, herpes zoster, viral infections of the upper respiratory tract);
- mental disorders (depression);
- from the central nervous system (dizziness, headache);
- from the respiratory system (pain in the throat and larynx, nasal congestion);
- from the gastrointestinal tract (diarrhea, vomiting);
- from the skin and subcutaneous tissues (itching);
- from the musculoskeletal system (back pain, myalgia, arthralgia);
- general disorders and reactions at the injection site (fatigue, erythema at the injection site, pain at the injection site, reactions at the injection site, including hemorrhage, hematoma, induration, swelling and itching);
- malignant neoplasms (non-melanoma skin cancer, malignant neoplasms of the prostate, intestines, mammary glands and melanoma in situ);
- hypersensitivity reactions (rash, urticaria).
Immunogenicity: Approximately 6% of patients receiving ustekinumab developed antibodies to the drug, which usually had a low titer. There was no clear correlation between the formation of antibodies and the presence of reactions with the injection. Most patients who had antibodies to ustekinumab also had antibodies that neutralized them. In the presence of antibodies to ustekinumab, patients often had lower efficacy of the drug, although the presence of antibodies does not exclude the achievement of a clinical effect.
In post-registration use of ustekinumab, adverse events from the immune system were identified: hypersensitivity reactions (including rash and urticaria), serious hypersensitivity reactions (including anaphylaxis and angioedema); from the skin and subcutaneous tissues: plaque psoriasis.
Pregnancy and lactation
It is not recommended to use the drug during pregnancy; effective methods of contraception should be used during and 15 weeks after treatment with the drug.
A decision should be made to stop breastfeeding while taking the drug or to discontinue ustekinumab therapy.
Contraindications
- Clinically significant hypersensitivity to ustekinumab or any excipient of the drug;
- Children's age (up to 12 years);
- Pregnancy and lactation;
- Serious infectious diseases in the acute phase, including tuberculosis;
- Malignant neoplasms.
Carefully
- Chronic or recurrent parasitic and infectious diseases of a viral, fungal or bacterial nature.
- History of malignant neoplasms.
- Elderly age.
Overdose
During clinical studies, patients were given single intravenous doses of up to 6 mg/kg without the development of dose-limiting toxicity. In case of overdose, it is recommended to monitor the patient's condition for signs and symptoms of side effects and, if they develop, appropriate symptomatic therapy should be started immediately.
special instructions
- Ustekinumab is a selective immunosuppressant and may increase the risk of developing infections and reactivation of latent infections. In clinical studies, serious bacterial, fungal and viral infections were observed in patients using ustekinumab. Ustekinumab should not be used in patients with clinically significant, active infections. Caution should be exercised when using the drug in patients with chronic infections or a history of recurrent infections.
- Before starting to use the drug, the patient should be tested for the presence of tuberculosis. Ustekinumab should not be used in patients with active tuberculosis. If you have latent or active tuberculosis (including a history of tuberculosis), treatment should be started before using ustekinumab. Treatment of tuberculosis should also be started in patients in whom the sufficient effect of previous treatment is unconfirmed. During and after treatment with ustekinumab, patients should be closely monitored for signs and symptoms of active tuberculosis.
- Patients should be warned to seek medical attention if signs and symptoms suggestive of infection appear. If a serious infection develops, the use of ustekinumab should be discontinued and the patient should be under the supervision of medical personnel. Ustekinumab should not be used until treatment for the infection has been completed.
- Immunosuppressants may increase the risk of developing malignancies. In some patients receiving ustekinumab in clinical trials, the occurrence of malignant neoplasms (cutaneous and non-cutaneous forms) was observed. The use of ustekinumab has not been studied in patients with a history of malignancy. Caution should be exercised when prescribing the drug to patients with a history of malignancy, as well as when considering continued treatment with ustekinumab in patients diagnosed with malignancy. All patients over the age of 60 years, as well as those who have previously received long-term therapy with immunosuppressants or UV radiation, should be screened for the presence of non-melanoma skin cancer.
- Serious hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during post-marketing use of ustekinumab. If anaphylactic or other serious hypersensitivity reactions occur, ustekinumab should be discontinued immediately and appropriate treatment should be instituted.
- The patient should not be vaccinated with live vaccines during the period of treatment with ustekinumab, as well as in the period 15 weeks before vaccination (after taking the last dose of the drug) and 2 weeks after vaccination. Caution should be exercised when using live vaccines to immunize family members of a patient receiving ustekinumab, since there is a risk of viral or bacterial release and transmission of infection from these individuals to patients. Long-term treatment with ustekinumab does not suppress the humoral immune response to vaccines containing pneumococcal polysaccharide and tetanus vaccine. Vaccines containing inactivated microorganisms can be used with ustekinumab, but the induced immune response may not be sufficient to prevent disease.
- Concomitant immunosuppressive therapy: The safety and effectiveness of ustekinumab in combination with immunosuppressive drugs and phototherapy have not been studied in patients with psoriasis. In studies in patients with psoriatic arthritis, coadministration with methotrexate did not affect the safety and efficacy of ustekinumab. Caution should be exercised when considering the possibility of simultaneous
- the use of other immunosuppressants and ustekinumab, as well as when switching from therapy with another antipsoriasis biological drug to ustekinumab therapy.
- Use in elderly patients (over 65 years): Of the 4031 patients treated with ustekinumab, 248 were patients over 65 years of age (183 patients with psoriasis and 65 with psoriatic arthritis). In clinical studies, there was no effect of age on clearance or volume of distribution of the drug. Although studies of the drug showed no differences in the safety and effectiveness of the drug in elderly patients over 65 years of age compared with younger patients, the number of elderly patients is insufficient to make a definitive conclusion about the effect of age (or lack thereof) on clinical effectiveness.
Use in children: The safety and effectiveness of ustekinumab in children has not been studied. The drug has not been studied in patients with renal or hepatic impairment.
Special instructions.
Currently, there is data on the effectiveness and safety of continuous therapy with ustekinumab for 5 years in patients with moderate to severe psoriasis. Multicenter, double-blind, randomized, placebo-controlled studies have shown that the effectiveness of ustekinumab remains high with continuous use of the drug for 5 years, and the most common adverse events are upper respiratory tract infections.
Prevention of palmoplantar psoriasis?
With the help of prevention, you can achieve a very long remission, which can last until the end of life. By eliminating risk factors from their lives, people who have sick relatives can avoid the risk of getting sick. Palmar and plantar psoriasis can be prevented by the following measures:
- maintaining a healthy, measured lifestyle; playing sports, frequent exposure to fresh air;
- proper regular nutrition;
- avoiding prolonged stress and heavy physical activity;
- complete cessation of alcohol and smoking;
- timely treatment of all acute and chronic diseases, elimination of foci of infection;
- prevention of palmar psoriasis, elimination of factors that irritate the palms - frequent work in water, contact with household chemicals and other chemicals;
- eliminating factors that irritate the soles - wearing tight shoes, skin infections of the feet;
- proper care of hands and feet - combating excessive sweating, applying emollient cosmetics.